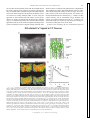
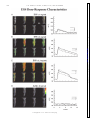
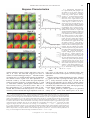
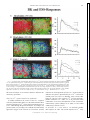
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Calcium Transients in the Garter Snake Vomeronasal Organ
Biological neuron model wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Binding problem wikipedia , lookup
Neural oscillation wikipedia , lookup
Mirror neuron wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Response priming wikipedia , lookup
Axon guidance wikipedia , lookup
Central pattern generator wikipedia , lookup
Synaptogenesis wikipedia , lookup
Nervous system network models wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Metastability in the brain wikipedia , lookup
Neural coding wikipedia , lookup
Development of the nervous system wikipedia , lookup
Electrophysiology wikipedia , lookup
Neuroanatomy wikipedia , lookup
Apical dendrite wikipedia , lookup
Circumventricular organs wikipedia , lookup
Single-unit recording wikipedia , lookup
Synaptic gating wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Multielectrode array wikipedia , lookup
Neural modeling fields wikipedia , lookup
Signal transduction wikipedia , lookup
Optogenetics wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Evoked potential wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Efficient coding hypothesis wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
J Neurophysiol 87: 1449 –1472, 2002; 10.1152/jn.00651.2001. Calcium Transients in the Garter Snake Vomeronasal Organ ANGEL R. CINELLI,1 DALTON WANG,2 PING CHEN,2 WEIMIN LIU,1 AND MIMI HALPERN1 Department of Anatomy and Cell Biology and 2Department of Biochemistry, State University of New York Downstate Medical Center, Brooklyn, New York 11203 1 Received 6 August 2001; accepted in final form 19 October 2001 The vomeronasal (VN) system of garter snakes is particularly well developed and is known to be critical for several species-specific behaviors including prey detection (Burghardt and Pruitt 1975; Graves and Duvall 1985; Halpern 1982, 1992; Halpern and Frumin 1979; Halpern and Kubie 1994; Kahmann 1932; Kubie and Halpern 1979; Naulleau 1965; Wilde 1938). The initial step in prey detection involves the interaction between a prey-derived chemoattractive ligand and specific receptors on the dendritic surfaces of bipolar neurons of the VN sensory epithelium. We have isolated and purified prey extracts and used them as selective chemoattractive stimuli. Among the compounds isolated are EW20 (Wang et al. 1988), a 20-kDa thiol-containing protein, EW3 (Wang et al. 1992, 1993), a low-molecular-weight chemoattractant [both derived from earthworm wash (EW)], and ES20 (Jiang et al. 1990), a 20-kDa glycoprotein derived from electric shock-induced earthworm secretion (ESS). ES20 specifically binds to the VN sensory epithelium in a saturable and reversible manner with a Kd of ⬃0.3 M (Jiang et al. 1990) and does not bind specifically to other organs, including brain and main olfactory epithelium. G proteins (Go, Gi, and Gs) have been immunologically detected in the VN sensory epithelium of garter snakes and ES20 receptors probably are coupled to these G proteins (Luo et al. 1994). Functional studies have demonstrated that ESS and ES20 evoke depolarizing currents in VN neurons and increase unit activity in the accessory olfactory bulb (AOB) mitral cells, the targets of the axons of receptor neurons of the VN epithelium (Jiang et al. 1990; Luo et al. 1994; Taniguchi et al. 1998, 2000). Binding of ES20 to VN receptors also results in increased levels of inositol 1,4,5-trisphosphate (IP3), suggesting that this signal cascade pathway may be involved in chemosensory transduction. In contrast, ES20 significantly reduces basal levels of cAMP as well as GTP␥S- or forskolin-induced high levels of cAMP (Luo et al. 1994). Nevertheless, an adenylate cyclase, ACVN, has been cloned from a garter snake VN epithelial library, which shows high homology to AC type VI (Liu et al. 1998) and is sensitive to Ca2⫹ regulation (Wang et al. 1997). Thus within the VN sensory epithelium components of two second-messenger systems, ACVN and phospholipase C (PLC), exist. In VN neurons, the reversal potential induced by dialysis of IP3 or its analogue 3-deoxy-3-fluoro IP3 mimics the reversal currents generated by the chemoattractive stimuli (Taniguchi et al. 2000). IP3, which is known to mobilize intracellularly sequestered Ca2⫹ via the IP3 receptor (IP3R), causes elevations of cytosolic Ca2⫹, which plays multiple functional roles in neurons including signal transduction (Berridge 1993; Clapham 1995). However, these events in VN neurons remain to be elucidated. Taking advantage in the garter snake of the presence of specific prey extracts as selective chemoattractant stimuli (ESS), in the present study, we disclose mechanisms triggering stimulus-induced cytosolic Ca2⫹ transients associated with chemosensory transduction. Optical recordings were performed in slices from VN neurons selectively loaded with the fluorescent Ca2⫹ indicator Calcium Green-1 from their axonal terminals in the AOB by retrograde transport. We report here that chemoattractants produce initially transient IP3-related ac- Address for reprint requests: M. Halpern, Dept. of Anatomy and Cell Biology, Box 5, SUNY Downstate Medical Center, 450 Clarkson Ave., Brooklyn, NY 11203 (E-mail: [email protected]). The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked ‘‘advertisement’’ in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. INTRODUCTION www.jn.org 0022-3077/02 $5.00 Copyright © 2002 The American Physiological Society 1449 Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 Cinelli, Angel R., Dalton Wang, Ping Chen, Weimin Liu, and Mimi Halpern. Calcium transients in the garter snake vomeronasal organ. J Neurophysiol 87: 1449 –1472, 2002; 10.1152/jn.00651.2001. The signaling cascade involved in chemosensory transduction in the VN organ is incompletely understood. In snakes, the response to nonvolatile prey chemicals is mediated by the vomeronasal (VN) system. Using optical techniques and fluorescent Ca2⫹ indicators, we found that prey-derived chemoattractants produce initially a transient cytosolic accumulation of [Ca2⫹]i in the dendritic regions of VN neurons via two pathways: Ca2⫹ release from IP3-sensitive intracellular stores and, to a lesser extent, Ca2⫹ influx through the plasma membrane. Both components seem to be dependent on IP3 production. Chemoattractants evoke a short-latency Ca2⫹ elevation even in the absence of extracellular Ca2⫹, suggesting that in snake VN neurons, Ca2⫹ release from intracellular stores is independent of a preceding Ca2⫹ influx, and both components are activated in parallel during early stages of chemosensory transduction. Once the response develops in apical dendritic segments, other mechanisms can also contribute to the amplification and modulation of these chemoattractantmediated cytosolic Ca2⫹ transients. In regions close to the cell bodies of the VN neurons, the activation of voltage-sensitive Ca2⫹ channels and a Ca2⫹-induced Ca2⫹ release from intracellular ryanodine-sensitive stores secondarily boost initial cytosolic Ca2⫹ elevations increasing their magnitude and durations. Return of intracellular Ca2⫹ to prestimulation levels appears to involve a Ca2⫹ extrusion mediated by a Na⫹/Ca2⫹ exchanger mechanism that probably plays an important role in limiting the magnitude and duration of the stimulation-induced Ca2⫹ transients. 1450 A. R. CINELLI, D. WANG, P. CHEN, W. LIU, AND M. HALPERN cumulation of [Ca2⫹]i in dendritic regions via two pathways: a Ca2⫹ release from IP3-sensitive intracellular stores and, to a lesser extent, a Ca2⫹ influx through the plasma membrane in dendritic regions. Although other mechanisms can secondarily participate in the modulation of these chemoattractant-induced cytosolic Ca2⫹ transients, cAMP seems not to have an important role in the generation these transient Ca2⫹ responses. METHODS Animals Adult garter snakes, Thamnophis sirtalis, of both sexes were obtained from commercial suppliers. They had all resided in the laboratory for ⱖ2 months prior to use in the experiments described here. VN bipolar neurons were loaded with Calcium Green 1 by means of retrograde axonal transport of dye placed in the accessory olfactory bulb (AOB). Snakes were anesthetized with a subcutaneous injection of methohexital sodium (0.5 mg/g body wt; Eli Lilly, Indianapolis, IN). A hole above the AOB was drilled through the skull to expose the AOB bilaterally, and Calcium Green 1 was applied either as crystals or a 5-l injection of Calcium Green solution injected deep into the glomerular layer. The snakes were allowed to recover for 3– 4 days prior to the evaluation of Ca2⫹-related fluorescence signals. Preparation of VN sensory epithelial tissue slices The methods are essentially similar to those described previously (Taniguchi et al. 1995, 1996, 2000). In brief, snakes were lightly anesthetized with methohexital sodium prior to decapitation. The vomeronasal neuroepithelium was dissected from the head after carefully removing the bony capsule and mushroom body, mounted onto a carrot block, and cut into slices, ⬃240 m thick, with a vibrating slicer (Vibratome 3000, Technical Products International, St. Louis, MO) in snake Ringer solution. This solution consisted of (in mM) 119 NaCl, 4.1 KCl, 2.5 CaCl2, 1.5 MgCl2, 15 glucose, 5 Na-pyruvate, and 10 HEPES (pH 7.4). The tissue slice was then mounted onto a small plastic petri dish. Preparation of ESS The methods are the same as described earlier by Jiang et al. (1990). Secretions were obtained by passing an electric current from a 9-V battery (20 6-s bursts with an intershock interval of 30 s) through the worms. A yellowish mucus-like secretion was collected and centrifuged, and the supernatant was dialyzed against distilled water. Preparation of VN sensory epithelial homogenate Essentially we followed a standard method as previously reported (Luo et al. 1994). Dissected VN epithelia were homogenized in cold buffer (20 mM Tris/HCl, pH 8, 1 mM PMSF, 80 g/ml DTT, 0.5 g/ml antipain, 1 g/ml leupeptin, 1 g/ml aprotinin, 0.6 g/ml chymostatin, and 0.6 g/ml pepstatin) and centrifuged at 500 g for 5 min. The supernatant was recovered and referred to as homogenate. Monitoring intracellular Ca2⫹ changes Video imaging of fluorescent changes was used to monitor calcium responses. The general plan of the optical setup is based on standard methods (Cinneli and Salzberg 1991, 1992; Cinelli OPTICAL SETUP. J Neurophysiol • VOL CALCIUM IMAGING TECHNIQUES. Fluorescence measurements of Ca2⫹ levels were performed following standard protocols. Data are reported as fractional changes over background fluorescence levels (F/Fo). Standard procedures for background subtraction and calibrations were used for calibration with solutions of known dye concentration (Tsien et al. 1985). After the experiments, in situ calibrations were performed. Cells were permeabilized with Ca2⫹ ionophores (ionomycin) or membrane solvents (digitonin or saponin). Fmax and Fmin were determined in Ringer solution (1 mM Ca2⫹) to saturate the Ca2⫹indicator and then by subsequently bathing the cells in low-Ca2⫹ Ringer solution supplemented with 5 mM EGTA. Calcium Green yielded increases in fluorescence signals proportional to Ca2⫹ bound; these levels were directly related to levels of [Ca2⫹]i. Other terms of the equation were assessed by in situ calibration (see following text). IMAGE PROCESSING. Images were digitized and stored in real time using a frame grabber board in a Pentium IBM-compatible computer system. Final images were analyzed by applying various digital filterings or convolution algorithms (Cinelli 1998, 2000; Cinelli and Salzberg 1991, 1992; Cinelli et al. 1995). High spatial resolution during image acquisition was necessary to preserve the image details in fine dendrites even when low band-pass spatial filters were used. Background experiments indicated that low band-pass spatial filters were often required to suppress pixel noise from the detector or the image intensifier. High spatial resolutions were also necessary for the application of deconvolution techniques after low band-pass spatial filters. Deconvolution techniques were used for improving focus resolution and obtaining better resolution for visualizing particular cellular compartments such as the dendritic terminals of vomeronasal neurons. Temporal plots of Ca2⫹ transients were obtained from averaged values over 8 ⫻ 8 pixel kernels. In the figures, changes over time are illustrated by pseudocolors resulting from subtracting basal levels of [Ca2⫹]i from those obtained after experimental manipulation. RESULTS Retrogradely labeled VN neurons Using slices of snake VN sensory epithelium, changes in cytosolic Ca2⫹ associated with chemosensory transduction were studied in VN neurons loaded with the Ca2⫹ indicator, Ca2⫹ Green 1. VN neurons were labeled by retrograde transport of this 87 • MARCH 2002 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 Loading VN bipolar neurons with Calcium Green 1 using retrograde axonal transport from the accessory olfactory bulb et al. 1995). Essentially the system consists of an upright epi-illumination microscope (Nikon Epiphot) with a video camera (MV-1070; Marshal Electronics) in the photographic port. Light from a 150-W xenon lamp (Optic Quip 1600) is collimated and rendered quasimonochromatic by one of several interference filters, focused by means of a quartz-UV-grade condenser (Nikon), and reflected to the preparation by a dichroic mirror. The wavelength for the excitation and emission filters and the dichroic mirror were selected according to the excitation and emission spectra of Calcium Green 1. To improve collection efficiency, fluorescent light from the cells was collected by high numerical aperture (n.a.) water-immersion objectives (⫻20 or ⫻40; Fluo; Nikon), which formed a real image on the CCD sensor of the video camera located in the image plane of the microscope. To further improve the sensitivity of this analog camera, image exposures were extended to increase light integration in the CCD sensor wells (Cinelli 1998). When fast acquisition rates were needed, an image intensifier was used to improve the sensitivity of the camera. Fluorescence emission usually remained constant during the experiments. To assure stability of the recordings and to avoid photobleaching effects, the excitation light levels were reduced by neutral density filters until the fluorescence intensity remained constant for 200 s of illumination. No significant levels of autofluorescence were observed in VN neurons, and the drugs, at the concentrations used, did not affect or quench fluorescence levels. CHEMOATTRACTANT-INDUCED CALCIUM RELEASE dye from their axonal terminals in the AOB. This method allowed the selective staining solely of mature VN neurons, which are the only cell elements in the vomeronasal organ (VNO) that send their axons to the AOB. Transport of the dye from the injection sites to VN neurons was usually obtained within 72– 84 h. Using this approach, we observed that not all VN neurons in a slice preparation were found to be labeled with the fluorescence indicator (Fig. 1A), a condition that probably arose from the rather localized application of the dye in the AOB. An important advantage of this system for obtaining selective retrograde staining is that the VNO 1451 and the AOB are in different and separated tissue compartments. This condition prevents the undesirable diffusion of the dye to the VNO and the nonspecific staining of other cell types. We consistently observed in the slices that only mature VN neurons were labeled with the fluorescence indicator (Fig. 1, A and B); no other cellular elements, such as sustentacular cells or immature VN neurons, were labeled. Therefore all of the Ca2⫹ responses evaluated in this study arose exclusively from fluorescence changes in VN neurons sending their axons to the AOB. In most of our recordings, the use of nonconfocal optics in Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 2⫹ FIG. 1. Ca transients in retrogradely labeled snake vomeronasal (VN) neurons. A shows a video image illustrating the selective staining of VN neurons with Ca2⫹ Green after retrograde transport of this dye from their axonal terminals in the accessory olfactory bulb (AOB). Observe the labeling in the cell bodies of mature VN neurons (red arrows) located in the intermediate region of the sensory epithelium. No other cellular elements, such as sustentacular cells or immature VN neurons were labeled. L, lumen; D, dendritic region; S, somata region; BL, basal lamina. Horizontal bar ⫽ 20 m. B: a schematic diagram showing the laminar organization of cellular elements in the snake VNO. As shown in A, the cell bodies of mature VN neurons (R) are located exclusively in the middle epithelial laminae with dendrites projecting toward the epithelial lumen. Adjacent to the basal lamina are basal cells (B); immature VN neurons (N) are located apical to the basal cells, and sustentacular cells (S) are located close to the luminal surface, intermixed with the dendrites of VN neurons. C: the increase in [Ca2⫹]i recorded in the 1st, 3rd, 5th, and 7th frames after elecrtic shock-induced earthworm secretion (ESS, 2.0 mg protein/ml) application. Records taken from a 16 video sequence (1 frame/s; 120-ms image exposure) showing the spatial distribution of Ca2⫹ responses. Abbreviations as in A. Optical signals were obtained from single runs, and are coded as follow: green: 5–10%; yellow: 11–15%; orange: 16 –20%; red: ⬎20%. In situ calibration performed after membrane permeabilization indicates that a 20% optical signal roughly corresponds to changes in cytosolic Ca2⫹ levels on the order of 500 nM. D: the time course of the increase in [Ca2⫹]i over baseline levels following ESS application from the entire sequence (16 frames) as determined from 3 different sites (8 ⫻ 8 pixels) shown in the first image in C (a– c). The 1st point in the plots corresponds to basal cytosolic values and the 2nd point to the first response obtained 1 s after stimulus application. Arrows indicate the times corresponding to the 4 images illustrated in C. J Neurophysiol • VOL 87 • MARCH 2002 • www.jn.org 1452 A. R. CINELLI, D. WANG, P. CHEN, W. LIU, AND M. HALPERN Resting Ca2⫹ levels Changes in cytosolic Ca2⫹ levels were determined with standardized optical-imaging techniques (Cinelli and Salzberg 1991, 1992; Cinelli et al. 1995). Using Calcium Green 1, single wavelength emission measurements of changes in fluorescence intensity represented estimates of [Ca2⫹]i variations. According to “in situ” calibrations performed at the end of the experiments (see METHODS), baseline [Ca2⫹]i was equivalent to 60.2 ⫾ 15.4 (SD) nM (n ⫽ 12), and cytosolic Ca2⫹ response peaks were in the range of 104 –740 nM elevations above resting levels, proportional to the intensity of stimulation. Experiments usually lasted 6 – 8 h. During this period, [Ca2⫹]i baseline levels remained steady (usually ⬍70 nM), and no deterioration of the preparations was observed as judged by the similarity of the Ca2⫹ responses obtained throughout the experimental sessions. Characteristics of chemoattractant-induced Ca2⫹ responses To determine whether VN stimulants evoke cytosolic Ca2⫹ changes associated with excitatory responses, VN epithelial slices (240 –300 m) were exposed to prey chemoattractant ESS while [Ca2⫹]i was measured. As illustrated in Fig. 1C, Ca2⫹ transients in VN neurons elicited by the bath application of ESS ligands consisted of sharp elevations in cytosolic Ca2⫹ J Neurophysiol • VOL levels that usually reached a peak amplitude within 1 s. Response peaks were followed by a brief plateau and then a more prolonged decay phase in which cytosolic Ca2⫹ levels gradually declined to baseline levels within 16 – 32 s. Ca2⫹ transients evoked by ESS ligands exhibited a rather widespread distribution. Figure 1, C and D, shows the spatial distribution and time course of typical Ca2⫹ transients evoked by bath application of ESS ligands (2.2 mg/ml). In general, patterns of activity displayed a scattered appearance with a heterogeneous organization in which it was possible to find nonuniform foci of activity distributed in multiple epithelial regions separated by silent sectors. Within each lamina there were important variations in the amplitude and time course of ESS responses, suggesting a different degree of activity among stimulated VN neurons. Usually Ca2⫹ signals from sectors showing the largest amplitudes exhibited the longest durations and lowest thresholds. Following successive stimuli at any given concentration of ESS, the overall organization of these Ca2⫹ responses among different epithelial sectors was rather constant. We interpret this finding as indicating that this heterogeneous pattern represents cumulative responses from dissimilar VN neurons responding in different degrees to the ESS ligands. ESS stimuli evoked clear dose-dependent Ca2⫹ transients in the concentration range of 0.5– 4.5 mg/ml protein. Figure 2, A–C, shows typical changes in kinetics and spatial distributions of Ca2⫹ responses following ESS stimuli applied at different concentrations. The concentration threshold for eliciting detectable Ca2⫹ increases with ESS in our slice preparation was between 0.8 and 1.2 mg/ml protein. These Ca2⫹-dependent fluorescence signals usually exhibited half-saturating peak responses at concentrations on the order of 7 mg/ml protein. In this study, most records were obtained with stimuli in the range of 2.2– 4.4 mg/ml protein, and these concentrations are similar to those that evoke clear electrophysiological responses in the VN epithelium (Taniguchi et al. 1998). As a control for the specificity of ESS ligands in eliciting Ca2⫹ responses associated to chemosensory transduction mechanisms, we evaluated the effect of actin which is a behaviorally inactive compound (unpublished observations). In contrast to the clear responses evoked by ESS, the application of actin to the bath solution evoked no detectable Ca2⫹ changes (Fig. 2D) even at relatively high concentrations (4 – 6 mg/ml). Once the stimulus threshold was reached, Ca2⫹ transients elicited by ESS exhibited relatively sharp response onsets with latency rise times in the range of 500 –750 ms according to temporal plots obtained from video image sequences acquired at 250 ms/image (data not shown in the figures). Sharp response onsets were also observed even when ESS stimuli were applied at the lowest concentration (0.75 mg/ml, Fig. 2A, plot). Higher ESS concentrations evoked Ca2⫹ signals that exhibited similar response profiles but larger amplitudes and longer durations. As shown in Fig. 2, A–C, the applications of increasing ESS concentrations evoked a proportional increase in the amplitude and duration of Ca2⫹ transients in all epithelial regions. There were also important changes in the spatial distribution of these responses as ESS concentration increased. At low concentrations (e.g., 1 mg/ml), there was a limited number of activated sites that were located almost entirely in dendritic regions. As ESS stimulus concentration increased, 87 • MARCH 2002 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 a rather thick slice preparation (240 –300 m) prevented us from obtaining, with certainty, Ca2⫹ signals at single-cell resolution. In fact, most of our optical recordings represented population responses from clusters of VN neurons. Despite this limitation, it was possible to study the spatial distribution of Ca2⫹ signals in different segments of VN neurons because the garter snake VNO exhibits a distinctive lamination pattern of cellular elements (Fig. 1B). In contrast to the less clear laminar organization of cellular elements found in other species (e.g., Halpern et al. 1995), the cell bodies of mature VN neurons are located exclusively in the middle epithelial laminae. Just above the basal lamina there are unstained cell elements corresponding to basal cells and immature VN neurons. In the present study, the latter were not stained because their axons had not reached the AOB. Sustentacular cells and the dendrites of VN neurons are located in the apical epithelial regions close to the luminal surface. As shown in Fig. 1B, these sustentacular cells were also unstained in our slices because first they lack projections to the AOB and second no diffusion of the dye occurred from the AOB to the VNO. Thus despite the lack of single-cell resolution in our recordings, the lamination pattern of the snake VNO and the specific staining of only VN neurons allowed us to define the source of the optical signals at least in three cellular compartments: the cell body region, the dendritic shaft region, and the apical dendritic region of VN neurons adjacent to the luminal surface. Consequently, as seen in all figure labels, Ca2⫹ responses recorded toward the base and in the middle of the epithelium corresponded to transients predominantly generated in or close to the cell bodies, optical signals from the upper VN epithelial sectors related primarily to Ca2⫹ transients from dendritic shafts, and fluorescence changes adjacent to the VN lumen corresponded to Ca2⫹ responses arising from the most apical segments of VN dendrites. CHEMOATTRACTANT-INDUCED CALCIUM RELEASE three major effects were observed in the spatial distribution of these responses. First, Ca2⫹ transients spread from dendritic locations to the cell body region of VN neurons where magnitudes and duration comparable to those found in dendritic regions were attained. Second, we observed a relative enlargement of individual foci of activity, involving adjacent epithelial sectors. Finally, new discontinuous foci of activity appeared in previously silent epithelial regions (Fig. 2, B and C). These new sites of activity did not overlap with other active sectors, suggesting the recruitment of a different set of active VN neurons. These results suggest that the newly active VN neurons were probably responding to distinct chemical cues present in ESS ligands that exhibited different response thresholds. Characteristics of different cytosolic Ca2⫹ transients in VN neurons J Neurophysiol • VOL could be activated following an initial Ca2⫹ influx mediated through VSCC, we evaluated the characteristics of Ca2⫹ transients elicited by high-[K⫹]o depolarization following the depletion of internal Ca2⫹ stores. Depletion of intracellular stores were obtained either using thapsigargin or ryanodine. Thapsigargin depletes intracellular Ca2⫹ store because it is a potent inhibitor of the intracellular Ca2⫹ pump and prevents Ca2⫹ reuptake into these pools following spontaneous or stimulusinduced depletions. As a consequence of this action, thapsigargin was used to determine the effect obtained after the depletion of all intracellular Ca2⫹ pools. Under our experimental conditions, we found that the action of thapsigargin (1 M for 10 –15 min) in depleting internal Ca2⫹ stores was predominantly stimulus dependent because the spontaneous Ca2⫹ depletion from these stores was rather low. As a consequence, the effect of thapsigargin was assessed following repetitive stimulation (see following text). Under these conditions, we observed that thapsigargin could shorten the time course and reduce the magnitude of K⫹-elicited Ca2⫹ signals, but this reduction was observed exclusively during the late phase of the response (n ⫽ 3; data not shown). Thus this result indicates that Ca2⫹ release enhanced the decay phase of VSCC-mediated signals. To further determine the involvement of ryanodine-sensitive pools in this effect, we used ryanodine instead of thapsigargin to selectively deplete these stores. Ryanodine acts as a selective antagonist of one of the two types of Ca2⫹ stores by forcing their release channels into a permanently opened state. For this purpose, we applied ryanodine (10 M) to the bath solution in the presence of high concentrations of caffeine (25 mM) for 10 –15 min. In all cases (n ⫽ 18), this protocol completely depleted ryanodine-sensitive Ca2⫹ stores as judged by the suppression of caffeine-elicited responses (as in other cell systems, we also found that in snake VN neurons Ca2⫹ transients evoked by caffeine depend exclusively on a Ca2⫹ release from ryanodine-sensitive stores; see following text). As illustrated in Fig. 4C, middle, following ryanodine treatment, K⫹-elicited Ca2⫹ signals exhibited a shortened time course and a reduction in magnitude during the late phases of the response. These changes were observed in both dendritic and somatic regions but were more obvious in regions adjacent to the cell bodies of VN neurons. At this level, Ca2⫹ transients elicited by K⫹ depolarizations exhibited a reduction during the decay phase equivalent to 20 –35% of control responses (n ⫽ 5). In no case was the initial onset or the peak amplitude of these responses affected. Thus K⫹ depolarization appears to elicit an initial Ca2⫹ influx through the activation of VSCC that in turn triggers a secondary Ca2⫹ release from ryanodinesensitive internal stores, potentiating and prolonging these responses. Therefore both thapsigargin and ryanodine actions indicate the presence of a CICR mechanism enhancing the late phases of the Ca2⫹ signals mediated through the activation of VSCC. CHARACTERISTICS OF CYTOSOLIC CA2⫹ ELEVATIONS ELICITED BY RELEASE FROM INTRACELLULAR STORES. To determine the characteristics of the Ca2⫹ transients in snake VN neurons that depend on Ca2⫹ release from internal stores, we evaluated the action of caffeine. By readily crossing the plasma membrane in different cell systems, caffeine evokes strong Ca2⫹ release, which depends exclusively on its reversible binding to intra- 87 • MARCH 2002 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 To elucidate possible mechanisms involved in the generation of ESS responses, we first established the general characteristics of different types of Ca2⫹ transients in VN neurons. Figure 3 compares the spatial distribution and kinetics of different types of Ca2⫹ signals evoked in snake VN neurons. Both ESS and caffeine (2–5 mM) applications gave rise to prolonged Ca2⫹ signals, which lasted 20 –50 s depending on the stimulus concentrations. These two responses, however, differed in their spatial distribution and onset characteristics. In contrast with ESS responses, caffeine signals were found adjacent to the cell body region, and the pattern of activation was more uniform across different regions. Caffeine signals also exhibited a slower rise time with a more progressive build-up, a characteristic that was evident especially at low concentrations (⬍2.5 mM; Fig. 3C). High [K⫹]o elicited Ca2⫹ transients that were relatively rapid in onset and brief in duration compared with other types of Ca2⫹ signals found in snake VN neurons (Fig. 3B). They were found predominantly in the cell body region and, as with caffeine-induced responses, were practically absent in apical dendritic segments. ROLE OF VOLTAGE-SENSITIVE CA2⫹ CHANNELS. To evaluate the role of voltage-sensitive Ca2⫹ channels (VSCC) in the generation of cytosolic Ca2⫹ changes, we determined the properties of Ca2⫹ responses elicited by high KCl (100 mM; Fig. 3B). The short duration and monotonic decay of these signals occurred even when high [K⫹]o was still present in the bath. This finding suggests a relatively rapid inactivation of VSCC in snake VN neurons. The total duration of these cytosolic Ca2⫹ changes was in the range of 3.5–5.5 s, lasting an average of 4.52 ⫾ 1.87 (SD) s (n ⫽ 7). Thus the time course of these transients were considerably shorter than those elicited by ESS. Response latencies were also shorter, usually shorter than the fastest time resolution tested in this study (250 ms/image). Response peaks attained fluorescent fractional changes equivalent to 25–32%, values that corresponded to [Ca2⫹]i elevation on the order of 175–290 nM. These Ca2⫹ signals were reversibly suppressed when extracellular Ca2⫹ was removed from the medium (Fig. 4A). They were also completely blocked after the application of common VSCC blockers such as cadmium (Cd2⫹; Fig. 4B) or cobalt (Co2⫹; 50 –100 M; data not shown), confirming that they resulted primarily from a Ca2⫹ influx through VSCC. To determine whether a Ca2⫹-induced Ca2⫹ release (CICR) 1453 1454 A. R. CINELLI, D. WANG, P. CHEN, W. LIU, AND M. HALPERN Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 J Neurophysiol • VOL 87 • MARCH 2002 • www.jn.org CHEMOATTRACTANT-INDUCED CALCIUM RELEASE 1455 cellular ryanodine receptors (Usachev and Thayer 1999; Usachev et al. 1993). We found that relatively low doses of caffeine (2.5 mM) could trigger strong Ca2⫹ transients in snake VN neurons (Fig. 3C). Similar to ESS responses, the amplitude and duration of these signals were dose dependent. Ca2⫹ transients evoked by 2.5 mM caffeine had peak amplitudes similar to responses evoked by 2.2 mg/ml ESS (Fig. 3, A and C). Under these conditions, optical signals exhibited fluorescence increases of 28.5 ⫾ 4.7% (⌬F/Fo; mean ⫾ SD; n ⫽ 8) that corresponded to [Ca2⫹]i elevations in the range of 190 – 200 nM. But in contrast to the ESS responses, Ca2⫹ transients elicited by caffeine attained their largest amplitudes in the cell body region of VN neurons. As we mentioned before, this distribution was similar to the responses elicited by high-[K⫹]o applications. Caffeine responses exhibited moderate rise times, followed by a plateau, and then a prolonged declining phase in which [Ca2⫹]i slowly returned to baseline (Figs. 3C and 5A). In contrast to ESS transients, the rise times of caffeine signals were highly dependent on stimulus concentration. At low concentrations, they exhibited a slow onset, which progressively became sharper at higher concentrations. Ca2⫹ signals elicited by caffeine appear to be generated entirely by Ca2⫹ release from internal pools. In all cases 2⫹ FIG. 2. General characteristics of Ca transients evoked by ESS ligands in garter snake VN neurons. A–C: patterns of activity show 8 consecutive records from 16 frame sequences illustrating [Ca2⫹]i over baseline levels, just before (1st frame) and following (remaining 7 frames) the application of ESS stimuli at 3 different concentrations (l, 2, and 3.3 mg protein/ml). Note that the luminal surface of the epithelium (L) is to the right, the basal lamina (BL) to the left. D: a similar sequence but with records obtained following the application of actin (3 mg/ml), which was used as a control and produced no detectable Ca2⫹ changes. Observe that in B and C, the increases in odorant concentrations evoked larger and more widespread responses over different regions of the vomeronasal sensory epithelium. Plots on the right illustrate the corresponding time course of Ca2⫹ signals from the whole sequence of images (16) at the 3 sites shown in A, 1st image (a– c are indicated by —, - - -, and 䡠 䡠 䡠 , respectively). In all cases, [Ca2⫹]i returns to baseline levels after 12–16 s, but higher concentrations produce longer response durations. Optical signals were obtained from single runs (1 frame/s, 120-ms image exposure). The 1st 2 points in the plots correspond to basal cytosolic values and the 3rd point to the 1st response obtained 1 s after stimulus application. Cytosolic Ca2⫹ levels were coded in pseudocolors as in Fig. 1C and overlapped on a fluorescent image of labeled VN neurons. — in D ⫽ 75 m. J Neurophysiol • VOL 87 • MARCH 2002 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 FIG. 3. Spatiotemporal characteristics of Ca2⫹ signals evoked by ESS (A), high K⫹ (B), and caffeine (C) in VN neurons. Patterns of activity in A–C correspond to 8 consecutive records from 16 frame sequences reflecting cytosolic Ca2⫹ elevations following stimulus applications. Right: plots correspond to the time course of Ca2⫹ signals from the whole sequence at 3 different epithelial sites shown in A, first image (a– c). Observe in all the responses the presence of broad patterns of activity spread throughout different epithelial sectors. Responses, however, had different spatial distributions. Note in A that not all retrogradely labeled receptor cells respond to ESS stimulation with increased Ca2⫹ signals. ESS signals reached their maximum in multiple, but confined, sectors in VN dendritic regions close to the epithelial lumen. In contrast, high-K⫹ and caffeine responses are found predominantly in proximity of the cell body region, a location where they attained their maximum peak values and longest durations. Observe also the differences in response kinetics; ESS and caffeine evoked Ca2⫹ signals having a long time course in comparison with the brief transient elevations evoked by high-K⫹ depolarization. They also exhibited more complex profiles during their decaying phases that contrast with the rather rapid monotonic decay of K⫹-elicited Ca2⫹ signals. ESS and high-K⫹ responses exhibited signals with sharp rise times, while Ca2⫹ responses evoked by caffeine have a slower onset, especially in small amplitude responses (see trace a). Optical signals were obtained from single runs (1 frame/s; 120-ms image exposure) and superimposed on a fluorescent image of the VN slice. The 1st point in the plots corresponds to basal cytosolic values and the 2nd point to the 1st response obtained 1 s after stimulus application. Cytosolic Ca2⫹ levels were coded as described in Fig. 1C. Abbreviations in Fig. 1A. Horizontal bar in C ⫽ 25 m. 1456 A. R. CINELLI, D. WANG, P. CHEN, W. LIU, AND M. HALPERN Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 2⫹ FIG. 4. Properties of Ca transients in snake VN neurons evoked by KCl (100 mM)-induced membrane depolarization. Plots a and b correspond to optical signals at 2 different sites in the cell body region of VN neurons. A: the effect of removing external Ca2⫹ from the medium on the Ca2⫹ transients; left: control responses; middle: the suppression of all Ca2⫹ signals following the removal of Ca2⫹ from the medium (0 [Ca2⫹]o supplemented with 5 mM EGTA); and right: recovery in normal Ringer solution. B: the action of cadmium, a general VSCC blocker, on the Ca2⫹ signals elicited by K⫹ depolarization. Left: Ca2⫹ signals under control conditions; middle: complete suppression of Ca2⫹ signals following the application of Cd2⫹ (50 M); and right: recovery in normal Ringer solution. C: the effect of depleting ryanodine-sensitive internal Ca2⫹ stores on K⫹-induced Ca2⫹ transients. Left: K⫹-induced Ca2⫹ signals under control conditions; middle: K⫹-induced Ca2⫹ signals obtained after the depletion of ryanodine stores. The depletion of ryanodine-sensitive stores shortens the duration of Ca2⫹ transients by selectively reducing the amplitude of the responses during their late decaying phases. Right: illustration of the lack of recovery of the late decaying response phase even after repetitive washings in normal Ringer solution, indicating an irreversible effect of ryanodine. Plots of Ca2⫹ signals were taken from single runs, 16 image sequences (1 frame/s; 120-ms image exposure). The 1st point in the plots corresponds to basal cytosolic values and the 2nd point to the first response obtained 1 s after stimulus application. studied (n ⫽ 7), the absence of Ca2⫹ from the medium (0 [Ca2⫹]o, supplemented with 5 mM EGTA) did not affect the characteristics of caffeine responses evaluated immediately J Neurophysiol • VOL after the Ca2⫹ removal. Under these conditions, Ca2⫹ responses exhibited profiles, time courses (14.8 ⫾ 2.3 s), and peak magnitudes (29.5 ⫾ 3.7 ⌬F/Fo) similar to controls (Fig. 87 • MARCH 2002 • www.jn.org CHEMOATTRACTANT-INDUCED CALCIUM RELEASE 1457 Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 2⫹ FIG. 5. Properties of Ca signals evoked by caffeine in snake VN neurons. A: the characteristics of caffeine responses obtained following the removal of Ca2⫹ from the medium. Left: corresponds to control responses obtained from a 4 ⫻ 4 pixel area in the layer of VN neuron somata. Middle: plots of Ca2⫹ signals elicted by caffeine (10 mM) following the removal of Ca2⫹ from the medium (0 [Ca2⫹]o with 5 mM EGTA). Although no significant change was observed in the initial response (—), successive caffeine applications evoked a use-dependent gradual reduction in consecutive Ca2⫹ signals, as shown in responses obtained after 4 and 8 caffeine applications (- - - and 䡠 䡠 䡠 , respectively). Right: the recovery of this Ca2⫹ transient after restitution of normal [Ca2⫹]o (2–3 min). B: the effect of depleting internal Ca2⫹ stores with thapsigargin on caffeine-elicited Ca2⫹ signals. Left: a control response; middle: traces correspond to the progressive reduction in these Ca2⫹ responses following 2, 4, and 8 caffeine stimulations after incubation of the slice with thapsigargin (1 M, 15 min). Observe that on the 8th trial, no detectable signals were obtained, and this suppression was not reversed following repetitive rinses with normal Ringer solution (right). C: Ca2⫹ signals evoked by caffeine following the depletion of ryanodine-sensitive internal stores. Left: corresponds to a control signal obtained before the application of ryanodine (20 M, 15 min). Middle: the absence of caffeine-induced Ca2⫹ transients after cytosolic Ca2⫹ levels returned to basal levels following ryanodine treatment (see further details in text). Similar to thapsigargin effects, the ryanodine suppression of optical signals did not recover after rinsing the preparation in normal Ringer solution. Tissue slices in B and C were tested in normal snake Ringer solution. Plots correspond to Ca2⫹ signals from the cell body region of VN neurons taken from a single 16 image sequence (1 frame/s; 120-ms image exposure). The 1st point in the plots corresponds to basal cytosolic values and the 2nd point to the 1st response obtained 1 s after stimulus application. J Neurophysiol • VOL 87 • MARCH 2002 • www.jn.org 1458 A. R. CINELLI, D. WANG, P. CHEN, W. LIU, AND M. HALPERN Role of the IP3 signaling cascade in the generation of Ca2⫹ transients Previous studies have suggested that phosphoinositide turnover leading to IP3 formation may constitute the second-messenger system mediating chemosensory transduction in the VN system (Holy et al. 2000; Kroner et al. 1996; Luo et al. 1994; Sasaki et al. 1999; Wekesa and Anholt 1997). Thus we were interested in determining whether the activation of this pathway could give rise to cytosolic Ca2⫹ changes similar to those elicited by ESS ligands. In different cell systems, bradykinin (BK) stimulates phosphoinositide turnover, which, in turn, among other actions, can increase production of IP3 (Kirischuk et al. 1995; Seymour-Laurent and Barish 1995; Verkhratsky and Kettenmann 1996). Thus it has been used rather extensively to evaluate the role of IP3 in intracellular Ca2⫹ signaling (see Berridge 1993, 1998). To determine the effect of BK in snake VNO and compare it to ESS ligands, we evaluated the J Neurophysiol • VOL actions of BK and ESS stimuli on IP3 levels in homogenates of the snake VN sensory epithelium. IP3 production was measured following protocols previously reported (Luo et al. 1994). VN homogenates (50 g protein) were incubated either with ESS (13 g) or BK (300 nM) in a reaction solution (500 l) of 25 nM Tris-acetate, pH 7.6, 5 mM MgAc2, 0.5 mM ATP, 1 mM DTT, 0.01 mM GTP, 0.1 mM CaCl2, and 0.1 mg/ml BSA). As a control, distilled water was used instead of ESS or BK. In all cases the incubation time was 1 min. Under these experimental conditions, ESS evoked IP3 levels equivalent to 210 ⫾ 10 (SD) pmol/mg proteins (n ⫽ 4) while BK evoked IP3 levels equivalent to 255 ⫾ 5.0 pmol/mg proteins (n ⫽ 4). These IP3 increases differed significantly from those obtained under control conditions [75 ⫾ 15 (SD) pmol/mg proteins; n ⫽ 4], suggesting that both ESS and BK induce similar phosphoinositide turnover leading to IP3 formation. To further determine whether these increases elicited by BK correspond to IP3 changes arising from VN neurons, IP3 levels were determined also in homogenates from VN preparations previously deafferented from the AOB. These VN preparations lack mature VN neurons because the sectioning of their axons induce their degeneration. In contrast with the intact VNO, in deafferented homogenates, BK and ESS incubations exhibited IP3 levels that did not differ significantly from those found under control conditions. Thus these data indicate that both ESS and BK increase IP3, and these increases take place predominantly in mature snake VN neurons. Ca2⫹ signals evoked by BK applications also exhibited strong similarities with the Ca2⫹ transients elicited by ESS ligands. As shown in Fig. 6, BK stimuli evoked Ca2⫹ transients that consisted of an initial sharp [Ca2⫹]i rise followed by a brief plateau and then a prolonged declining phase in which cytosolic Ca2⫹ levels slowly returned to basal values, with a halftime in the range of 9 –14 s, depending on the stimulus concentration. BK responses were also dose dependent in the concentration range tested in this study (50 –300 nM) with amplitudes and durations directly proportional to stimulus intensity. Application of 200 nM of BK evoked peak fluorescent signals attaining fractional changes (⌬F/Fo) equivalent to 25– 40%. These values roughly corresponded to elevation in [Ca2⫹]i on the order of 150 –390 nM. Under these conditions, Ca2⫹ signals exhibited a time course in the range of 12–16 s. Like ESS responses, BK signals exhibited relatively sharp rise times even at low concentrations (Fig. 6B), characteristics that differed from the Ca2⫹ responses elicited by caffeine (Fig. 3C). Ca2⫹ transients elicited by ESS and BK also shared a similar spatial distribution with responses distributed in somatic as well as in dendritic sectors (Fig. 6, A and C). This presence of Ca2⫹ signals in dendritic regions, even those adjacent to the luminal surface, differed from the localization of caffeine responses found largely in the cell body region of VN neurons. Despite their similarities, we found some differences between the overall distribution of BK and ESS elicited Ca2⫹ signals. Unlike ESS responses, BK patterns of activity were more uniformly distributed across different sectors of the epithelium, and lacked the multifocal appearance characteristic of ESS responses. As illustrated in Fig. 6, even at relatively low concentrations, BK-evoked responses were homogeneously distributed over relatively large epithelial regions. This more uniform distribution probably reflects a lack of selectivity by 87 • MARCH 2002 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 5A, middle, 1st trace). However, we consistently found that successive applications of caffeine in Ca2⫹-free medium evoked Ca2⫹ transients that exhibited a progressive reduction, first in their amplitude and then in their duration (n ⫽ 7; Fig. 5A, middle, - - - and 䡠 䡠 䡠 ). The most likely explanation for this result is that these reductions are a consequence of the inability of intracellular Ca2⫹ stores to be fully refilled in Ca2⫹-free medium (Usachev and Thayer 1999). This interpretation is corroborated by the finding that there was a complete recovery of caffeine responses when normal [Ca2⫹]o was restored to the medium (Fig. 5A, right). Further confirmation about the role of intracellular Ca2⫹ release in the generation of caffeine responses was obtained by evaluating these signals after thapsigargin or ryanodine treatment. Both thapsigargin (1 M; Fig. 5B) and ryanodine (20 M; Fig. 5C) suppressed caffeine-induced Ca2⫹ transients but with some differences in their actions. VN slices preincubated with thapsigargin (10 –15 min) evoked no major changes in basal cytosolic Ca2⫹ levels, and initial caffeine applications elicited Ca2⫹ signals that exhibited only a slight reduction in their amplitude and duration. Under these conditions, however, successive caffeine applications evoked a progressive reduction in magnitude and duration of Ca2⫹ transients that led, after 8 –15 trials, to a complete response suppression (Fig. 5A, series of traces in the middle plot). No major change was observed using different intervals between trials (n ⫽ 5). In contrast, the magnitude of these reductions was use-dependent because it was related to the magnitude and duration of the previous caffeine responses. Altogether this evidence suggests that the reduction and eventual suppression of caffeine responses were caused by the gradual and irreversible depletion of internal Ca2⫹ stores. Ryanodine depletion also suppressed caffeine-elicited Ca2⫹ signals (Fig. 5C). But in contrast to the gradual effects observed with thapsigargin, the protocol used here to deplete ryanodine-sensitive stores (see preceding text) evoked a complete suppression of all caffeine responses from the first trial (Fig. 5C, middle and right, respectively). This irreversible suppression of caffeine responses following ryanodine treatment not only confirms that these Ca2⫹ transients arise entirely from internal Ca2⫹ release but further indicates that this release depends primarily on ryanodine-sensitive pools. CHEMOATTRACTANT-INDUCED CALCIUM RELEASE 1459 BK in the activation of VN neurons that have different chemosensory specificities. Source of Ca2⫹ signals related to IP3 production To further characterize the sources of the Ca2⫹ responses evoked by ESS and BK stimuli, we determined whether these responses depend on a Ca2⫹ release from intracellular stores or a Ca2⫹ influx through the plasma membrane. According to our present results (see preceding text), both BK and ESS stimuli appeared to trigger an IP3 increase in VN neurons, and this J Neurophysiol • VOL molecule can elicit important cytosolic Ca2⫹ elevations that, in different cell systems, depend entirely on a Ca2⫹ release from internal pools (for review, see Berridge 1998). On the other hand, IP3 can also evoke cytosolic Ca2⫹ increases through a plasma membrane Ca2⫹ influx via an IP3-activated cation conductance as has been demonstrated in some invertebrate chemosensory systems (Munger et al. 2000; see also Schild and Restrepo 1998 for review). BK RESPONSES IN CA2⫹-FREE MEDIUM. To determine whether BK responses in snake VN neurons depend on Ca2⫹ entry 87 • MARCH 2002 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 2⫹ FIG. 6. Comparison of the spatiotemporal characteristics of Ca signals evoked by ESS and the IP3 agonist bradykinin (BK). A–C: the spatial distribution of Ca2⫹ signals during the peak amplitudes (left) and the decaying phase of these responses (right, 8th frame). Observe that the Ca2⫹ signals elicited by BK (A and B) were distributed more evenly among different epithelial sectors than those evoked by ESS (C). Right: plots correspond to the time course of the Ca2⫹ signals obtained at 3 different locations shown in the 1st image in A. Optical signals were obtained from single runs of 16 image sequences (1 frame/s; 120-ms image exposure). The 1st point in the plots corresponds to basal cytosolic values and the 2nd point to the 1st response obtained 1 s after stimulus application. Cytosolic Ca2⫹ levels were coded as in Fig. 1C. Abbreviations as in Fig. 1. Horizontal bar in C ⫽ 25 m. 1460 A. R. CINELLI, D. WANG, P. CHEN, W. LIU, AND M. HALPERN To further characterize the component of BK responses that was independent of Ca2⫹ release, we evaluated these signals after the depletion intracellular Ca2⫹ stores. As with Ca2⫹ transients evoked by caffeine, the preincubation of VN slices with thapsigargin (1 M for 10 –15 min) did not affect initial BK responses to a large extent (Fig. 8B). Subsequent BK applications, however, evoked a progressive decrease affecting both the amplitude and duration of these Ca2⫹ signals. These reductions were observed equally in all epithelial regions and depended on the stimulus concentration and the number of previous stimulations. There was, however, some difference in the evolution of these changes as repetitive BK stimuli were delivered. In regions corresponding to the cell bodies of VN neurons, cuBK RESPONSES AFTER DEPLETING CA2⫹ STORES. J Neurophysiol • VOL 2⫹ FIG. 7. Characteristics of Ca signals evoked in apical dendritic regions of VN neurons by BK in the absence of [Ca2⫹]o. A: responses to BK (300 nM) obtained in normal Ringer solution (control) and immediately following removal of Ca2⫹ from the bath {0 [Ca2⫹]o with 2 mM EGTA (1st trial)}. Observe the rapid reduction in the peak amplitude of the Ca2⫹ signal that occurred without delay after removing extracellular Ca2⫹. B: the effect of repetitive BK applications in Ca2⫹-free medium. After their initial reduction, Ca2⫹ responses in dendritic regions maintained rather constant characteristics for a few runs (2–5) and progressively decreased following subsequent BK applications. This cumulative decrease gradually affected both the amplitude and duration of Ca2⫹ responses until all cytosolic Ca2⫹ changes become undetectable (usually after 20 runs using this stimulus concentration; bottom trace). C: full recovery shown of BK-elicited Ca2⫹ signals after the VN slices were returned to normal Ringer solution for 5 min. All Ca2⫹ signals were taken from single runs of 16 image sequences (1 frame/s; 120-ms image exposure). The 1st point in the plots corresponds to basal cytosolic values and the 2nd point to the 1st response obtained 1 s after stimulus application. mulative reduction progressed until all Ca2⫹ responses elicited by BK were abolished. Depending on the stimulus concentration, this occurred usually after 8 –20 consecutive applications of BK. In apical dendritic regions, however, responses failed to be completely suppressed. Instead, the reduction progressed until it revealed a small component that remained insensitive to 87 • MARCH 2002 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 through the plasma membrane or a release from internal pools, we first evaluated these responses in [Ca2⫹]o-free medium. In general, the absence of extracellular Ca2⫹ did not abolish BK responses, suggesting that these signals depend largely on a Ca2⫹ release from internal stores. In all the slices evaluated (n ⫽ 6), applications of BK (100 –200 nM) in 0 [Ca2⫹]o medium (supplemented with 2 mM EGTA) evoked Ca2⫹ signals that exhibited similar characteristics and kinetics to control responses. Under these conditions, most Ca2⫹ transients preserved their relatively sharp onsets, magnitudes, and durations. Although in most epithelial regions there was no modification, Ca2⫹-free conditions affected BK signals in apical dendritic regions close to the luminal surface of the epithelium. In this sector, we consistently observed that immediately after the removal of Ca2⫹ from the bath there was a sudden but constant reduction in the magnitude of BK responses equivalent to a decrease of 10 –18%. These decreases were observed in the amplitude at the peak of the responses as well as during their early decay phase. These changes are illustrated in Fig. 7A, which shows BK signals recorded in apical epithelial regions before (—) and after (- - -) the removal of Ca2⫹ from the medium. Full response recovery was always obtained without delay after the restitution of normal [Ca2⫹]o in the bath. This evidence indicates that Ca2⫹ signals elicited by BK stimuli in these apical dendritic segments of VN neurons has a component generated by a Ca2⫹ influx through the plasma membrane. In this region, however, there is still a response remaining that is unaffected by removal of Ca2⫹ from the medium. This component, present in Ca2⫹-free medium, appears to be largely dependent on Ca2⫹ release from intracellular stores as is the case for BK-evoked signals obtained in more basal regions. In contrast with the initial, sudden reduction found in apical dendritic regions, a different type of response decrease was found in Ca2⫹-free medium following repetitive BK stimulations (trials 8 –20). These changes were observed in all epithelial regions, including the apical dendritic locations, and consisted of a progressive and cumulative decrease in response magnitude and duration following subsequent applications of BK (6 –20; Fig. 7B). The degree of reduction in these Ca2⫹ signals was dependent on the number and the concentration of previous BK applications. As was the case for Ca2⫹ transients elicited by repetitive applications of caffeine when the VN slices were in Ca2⫹-free medium, these reductions probably arise from the progressive inability of intracellular Ca2⫹ stores to be replenished in 0 [Ca2⫹]o. CHEMOATTRACTANT-INDUCED CALCIUM RELEASE 1461 thapsigargin (n ⫽ 7) even after multiple BK applications (12–25 trials). This component attained its largest amplitude in locations adjacent to the epithelial luminal surface with a magnitude equivalent to 15–24% of the control responses and a duration of ⬃4 – 6 s. The real amplitude and duration of these Ca2⫹ transients, however, could be underestimated in our recordings because it appears to be generated in a highly localized dendritic region that cannot be independently assessed due to the population nature of our optical signals. Figure 8A illustrates the characteristics of BK responses recorded in apical dendritic regions after the depletion of internal Ca2⫹ stores by thapsigargin. As seen in the middle plots, BK applications in thapsigargin-treated slices evoked a reduction of Ca2⫹ signals, and after the sixth trial, it is possible to distinguish the emergence of a thapsigargin-resistant component, which maintained a rather constant magnitude and J Neurophysiol • VOL duration during subsequent stimulus applications (12–20 trials; middle and right). Under this condition, caffeine applications failed to evoke any detectable [Ca2⫹]i changes in responses recorded in the soma region (data not shown), indicating that this dendritic component does not depend on a partial depletion of intracellular Ca2⫹ stores. In contrast, the removal of Ca2⫹ from the medium reversibly abolished this thapsigargin-resistant component. Thus altogether this evidence indicates that this component is not generated by an internal Ca2⫹ release but instead is dependent on a Ca2⫹ influx through the plasma membrane. Confirming this notion, the magnitude and duration of this component matches the initial reductions in control BK responses observed in the same region when Ca2⫹ was removed from the medium (Fig. 7A). In contrast with thapsigargin, ryanodine-induced Ca2⫹ depletion did not greatly affect the characteristics of Ca2⫹ tran- 87 • MARCH 2002 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 2⫹ FIG. 8. Ca signals recorded in apical dendritic regions of VN neurons evoked by BK following depletion of internal Ca2⫹ stores. A: the effects of depleting internal Ca2⫹ stores on responses following the preincubation of a VN slice with thapsigargin (1 M). Left: Ca2⫹ signals under control conditions. Middle: traces illustrate Ca2⫹ responses evoked by multiple BK stimuli tested 15 min after the application of thapsigargin; note the reduction, but not total suppression, of these Ca2⫹ signals. Right: the thapsigargin-resistant portion of the original BK response remained constant after 20 trials. B: Ca2⫹ signals in dendritic and somatic regions evoked by BK after the depletion of ryanodine-sensitive stores. Left: BK responses under control conditions. Middle: Ca2⫹ signals obtained after the depletion of ryanodine stores (see protocol in the text). Observe that the Ca2⫹ signal from dendritic locations shows no detectable changes, while that from the somata region exhibits a significant reduction during its decaying phase. Right: after ryanodine treatment there was no detectable Ca2⫹ signal evoked by caffeine stimulation, corroborating the full depletion of ryanodine-sensitive stores under this condition. Ca2⫹ signals obtained from single runs of 16 image sequences (1 frame/s; 120-ms image exposure). The 1st point in the plots corresponds to basal cytosolic values and the 2nd point to the 1st response obtained 1 s after stimulus application. 1462 A. R. CINELLI, D. WANG, P. CHEN, W. LIU, AND M. HALPERN ESS RESPONSES IN CA2⫹-FREE MEDIUM. The mechanisms involved in the generation of Ca2⫹ signals elicited by ESS ligands were analyzed using protocols similar to those used with BK stimulation. First, we evaluated whether Ca2⫹ transients evoked by ESS ligands depend on Ca2⫹ influx or reflect a release from internal stores. As with BK signals we found that the absence of Ca2⫹ in the medium (0 [Ca2⫹]o, supplemented with 5 mM EGTA) did not suppress ESS responses. Immediately after the removal of Ca2⫹ from the medium, Ca2⫹ signals in most epithelial locations exhibited kinetics and characteristics similar to control responses. Ca2⫹ signals obtained in apical dendritic regions adjacent to the epithelial lumen, however, exhibited a slight reduction in their peak amplitudes of ⬃12–20% (Fig. 9B). As observed with reductions in BK responses, the late phase of these responses was less affected. These changes were also fully reversible. In contrast with these local and immediate effects, repetitive ESS stimuli under Ca2⫹-free conditions evoked a different type of response reduction. As with the effects found in BK signals, numerous ESS applications (8 –20 trials) in Ca2⫹-free medium evoked a progressive decrease in Ca2⫹ signals in all epithelial regions. These reductions were use dependent because they were related to the number and concentration of preceding stimulations and affected both the size and duration of the J Neurophysiol • VOL 2⫹ FIG. 9. Characteristics of Ca signals in apical dendritic regions of snake VN neurons evoked by ESS ligand in the absence of [Ca2⫹]o. A: traces correspond to Ca2⫹ responses elicited by ESS (2.2 mg/ml) under control conditions and immediately after the removal of Ca2⫹ from the bath (0 [Ca2⫹]o with 2 mM EGTA). Observe the immediate reduction in the peak amplitude and initial decaying phase of the response. B: superimposed traces illustrate decremental Ca2⫹ signals evoked by successive ESS applications in VN slices maintained in Ca2⫹-free medium. Like BK responses illustrated in Fig. 7, following the initial decrease, successive ESS applications evoke a progressive response reduction that affected both the amplitude and duration of Ca2⫹ signals, until these cytosolic Ca2⫹ elevations become undetectable (usually after 16 –20 runs using this stimulus concentration; bottom trace). C: full recovery of ESS-evoked Ca2⫹ signals after the VN slices were returned to normal Ringer solution for 5 min. Ca2⫹ signals were obtained from single runs of 16 image sequences (1 frame/s; 120-ms image exposure). The 1st point in the plots corresponds to basal cytosolic values and the second point to the 1st response obtained 1 s after stimulus application. Ca2⫹ signals until they disappeared. Figure 9B illustrates the progressive decay of ESS responses following repeated stimulation in Ca2⫹-free medium. This type of change usually appeared after the fourth ESS application, and following the eighth stimulus, Ca2⫹ signals started to exhibit major reductions in peak amplitude (70 – 85%) and duration. At this stage, Ca2⫹ transients also exhibited slower rise times and longer 87 • MARCH 2002 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 sients elicited by BK stimuli. After Ca2⫹ depletion from ryanodine stores (application of 10 M ryanodine with high concentrations of caffeine, 25 mM), Ca2⫹ transients elicited by BK applications were affected significantly only in the cell body region of the epithelium (Fig. 8B, middle, - - -). But even changes in this region were limited because they consisted of only slight reductions in peak amplitudes (equivalent to 12– 18%) and a minor shortening in duration (in the range of 30%) of these responses. Figure 8B illustrates typical Ca2⫹ signals evoked in dendritic (—) and somatic regions (- - -) before (left) and after (middle) the depletion of ryanodine-sensitive stores. Practically no change is observed in the signals evoked by BK in dendritic regions (a), and the Ca2⫹ signals from the somatic regions (b) exhibited only a slight reduction in magnitude, which occurred predominantly during its decay phase. Under these condition, however, caffeine applications (2–5 mM) evoked no detectable Ca2⫹ signals (Fig. 8B, right), indicating that the lack of major changes in BK responses was not caused by an incomplete depletion of ryanodine stores. Therefore these data suggest that BK responses (especially in dendritic regions) depend largely on a Ca2⫹ release from nonryanodinesensitive pools, probably IP3-sensitive stores. Furthermore, the relative lack of action of ryanodine on these BK-induced Ca2⫹ signals indicates that at least in dendritic regions a functional separation between ryanodine- and nonryanodine-sensitive Ca2⫹ pools probably exists. Assuming that this interpretation is correct, the reduction observed in somatic regions after the depletion of ryanodine-sensitive stores might be secondary and reflect an impairment in CICR mechanisms, which perhaps participate in amplifying BK responses. This interpretation is consistent with the observation that the effect is predominantly in the decaying portion of these responses. This hypothesis is also consistent with the reduction in the Ca2⫹ signals evoked by K⫹ depolarization observed following the depletion of ryanodine-sensitive stores (see preceding text; Fig. 4C). CHEMOATTRACTANT-INDUCED CALCIUM RELEASE peak latencies. A complete recovery of ESS responses was observed after 5–10 min incubation in normal [Ca2⫹]o (Fig. 9C). As with similar use-dependent reductions found in caffeine and BK responses, this progressive response decrease following repetitive ESS stimulation in Ca2⫹-free medium probably reflects the inability of intracellular Ca2⫹stores to be replenished. ESS RESPONSES AFTER THE DEPLETION OF CA2⫹ STORES. To confirm the characteristics of the components dependent and independent of internal Ca2⫹ release, ESS responses were evaluated after the depletion of these intracellular stores. As with BK signals, following the application of ESS ligands, VN slices preincubated with thapsigargin (1 M for 10 –15 min) 1463 evoked Ca2⫹ signals that exhibited a gradual reduction in amplitude and duration, consistent with a progressive depletion of internal Ca2⫹ stores (Fig. 10A). In the cell body region, ESS responses were completely suppressed by multiple ESS applications (8 –10 trials), indicating that at this level these responses depend entirely on Ca2⫹ release from internal stores. In apical dendritic regions, however, the depletion induced by thapsigargin failed to completely suppress all Ca2⫹ signals, even following numerous applications of ESS ligands (⬎20 trials). As with BK responses, there was a thapsigargin-resistant component that was revealed after the disappearance of the overlapping ESS response dependent on intracellular Ca2⫹ release (Fig. 10A). Fig. 10A, right, shows that this ESS-evoked J Neurophysiol • VOL 87 • MARCH 2002 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 2⫹ FIG. 10. Spatiotemporal properties of Ca signals in VN neurons evoked by ESS after the depletion of internal Ca2⫹ stores. A: the effects on ESS responses of depleting internal Ca2⫹ stores with thapsigargin (1 M, 15 min). Left: the image and the plot correspond to Ca2⫹ signals under control conditions. Middle: traces illustrate the use-dependent reduction in the Ca2⫹ signals evoked by successive applications of ESS after thapsigargin treatment (15 min). The top image of the right panel illustrates the distribution of Ca2⫹ signals in a thapsigargin-treated slice after 10 ESS stimulations, and the lower plot (right panel) shows the presence of this component from the apical dendritic region even after 20 trials. Top images correspond to the distribution of activity at the signal peaks, and lower plots illustrate the time course of Ca2⫹ signals as recorded from the apical dendritic region (E in the 1st image). B: the characteristics of ESS responses after the depletion of ryanodine-sensitive stores. Left: the image and the plots correspond to Ca2⫹ signals under control conditions; right: image and plots correspond to responses obtained after the depletion of ryanodine stores (20 M ryanodine; see protocol in the text). The top images illustrate the spatial distribution of responses at their peaks, while the lower plots show the time course and amplitude of Ca2⫹ signals from somatic (a) and dendritic (b) regions. Observe that Ca2⫹ signals in dendritic locations (- - -) show no detectable changes, while the amplitude and duration of the response from the somata region (—) was affected predominantly during its late phase. Optical signals were obtained from single runs (1 frame/s; 120-ms image exposure). The 1st point in the plots corresponds to basal cytosolic values and the 2nd point to the 1st response obtained 1 s after stimulus application. Cytosolic Ca2⫹ levels were coded in pseudocolors as in Fig. 1C. Abbreviations as in Fig. 1. Horizontal bar in B ⫽ 25 m. 1464 A. R. CINELLI, D. WANG, P. CHEN, W. LIU, AND M. HALPERN Possible role of cAMP in responses to ESS In the main olfactory system, odor transduction and associated Ca2⫹ transients rely on the activation of a cAMP pathway. The binding of odorants to olfactory receptors causes a Gprotein activation of adenylate cyclase, which, via cAMP, activates a cyclic nucleotide-gated (CNG) channel. The opening of this channel generates an inward transduction current carried by Na⫹ and Ca2⫹, and this influx is largely responsible for the Ca2⫹ transients elicited during odor stimulation. To determine whether similar CNG channel activity participates in a Ca2⫹ influx in snake VN neurons, we evaluated ESS responses after the application of the specific CNG channel blockers LY 83583 and L-cis-diltiazem (LCD), which are known to abolish Ca2⫹-related odor responses in olfactory receptor neurons (ORNs) (Kolesnikov et al. 1990; LeindersZufall et al. 1997, 1998). As illustrated in Fig. 11, preincubation of VN slices with either LY 83583 (80 M; B) or LCD (40 M; C) for 10 –15 min did not affect the general spatial distribution (Fig. 11, B and C) or the kinetics (Fig. 11E) of Ca2⫹ transient elicited by ESS ligands. The amplitudes, latenJ Neurophysiol • VOL cies, and time courses of ESS responses remained practically unchanged after the application of either of these CNG channel blockers (Fig. 11E). A similar absence of effect was observed in all the slices evaluated (n ⫽ 5). Furthermore, no changes were observed even in the dendritic regions adjacent to the luminal surface of the epithelium where we found a component arising from Ca2⫹ influx. To eliminate the possibility that in VN neurons ESS responses are mediated by CNG channels that are insensitive to the blockers tested or by the participation of an unknown cAMP-related mechanisms, we evaluated the effects of forskolin on cytosolic Ca2⫹ changes in these cells. As in other cell systems, forskolin appears to act as an adenylate cyclase (AC) activator directly inducing an increase in cAMP levels in VN neurons (Luo et al. 1994; Wang et al. 1997). Despite this action, we found that the addition of forskolin (10 M) to the bath evoked no detectable [Ca2⫹]i changes, at least in the time frame in which Ca2⫹ responses were analyzed in this study (n ⫽ 5; Fig. 10F). Thus altogether these results indicate that cAMP mechanisms do not directly participate in mediating the Ca2⫹ transients that occur during the early phases of chemosensory transduction in snake VN neurons. Role of Na⫹/Ca2⫹ exchanger in ESS responses We also investigated whether the Ca2⫹ influx observed during ESS response was dependent on a reverse mode of operation of the Na⫹/Ca2⫹ exchanger. In different cell systems, the operation of the Na⫹/Ca2⫹ exchanger is fully reversible and can mediate either a Ca2⫹ influx or efflux depending on the ionic gradients present across the plasma membrane (DiPolo and Beauge 1987, 1999). Thus it is conceivable that during VN chemosensory transduction an increase in cytosolic Na⫹ could activate the Na⫹/Ca2⫹ exchanger in its reverse mode forcing Ca2⫹ into the cell as has been reported recently in squid (Danaceau and Lucero 2000). To characterize the functional role of the Na⫹/Ca2⫹ exchanger in VN neurons, we evaluated ESS responses under conditions that inhibit its activity. Because monovalent cations cannot substitute for Na⫹ on the Na⫹/Ca2⫹ exchanger (Dipolo and Beauge 1987, 1999; Fiero et al. 1998), we blocked the exchanger by replacing external Na⫹ with Li⫹ or choline. After the substitution of Na⫹ by equimolar concentrations of Li⫹ or choline, we observed no permanent changes in basal [Ca2⫹]i. In some cases (n ⫽ 3), however, immediately after the substitution of [Na⫹]o we observed increases in cytosolic Ca2⫹ that returned to normal values within 1–2 min. We interpret these Ca2⫹ elevations as reflecting a brief Ca2⫹ influx through the exchanger operating in reverse mode following a sharp depletion of [Na⫹]i. During ESS responses, however, we found that the Na⫹/Ca2⫹ exchanger operates in the forward mode, mediating instead a Ca2⫹ efflux. In all the cases studied (n ⫽ 6), we found that the blockade of exchanger activity following the replacement of Na⫹ by Li⫹ enhanced ESS responses. Under this condition, Ca2⫹ signals exhibited slight increases in their peak amplitude, and major increases in their duration and magnitude. As seen in Fig. 12B, the changes in amplitudes were more prominent during the late phases of ESS responses. These Ca2⫹ signals exhibited longer plateaus and slower decay rates, characteristics that increased the total duration of these transients by 35–50%. Because Li⫹ may 87 • MARCH 2002 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 component persisted after numerous stimulus applications (20 trials). This component was, however, reversibly suppressed in Ca2⫹-free medium, supporting the notion that it arises directly from Ca2⫹ influx through the plasma membrane. This remaining ESS response exhibited its largest amplitudes in locations just adjacent to the luminal surface of the epithelium (Fig. 10A, right), similar to the BK-induced response. In this location, the response attained a magnitude equivalent to 15–20% of the full response obtained before the thapsigargin-induced Ca2⫹ depletion. However, as we previously mentioned for the similar component in BK signals, the actual size of this Ca2⫹ influx could be underestimated in our population optical recordings due to the difficulty of accurately determining cytosolic Ca2⫹ levels from localized regions. These data indicate that Ca2⫹ transients associated with chemosensory transduction in VN neurons depend on two different mechanisms: a widespread component generated by Ca2⫹ release from intracellular stores and a more restricted component that depends on Ca2⫹ influx in apical dendritic regions. In addition, the strong similarities between the kinetics and properties of BK and ESS responses suggest that both Ca2⫹ transients are closely interrelated, probably sharing similar mechanisms linked to PLC activation and IP3 production. Because we found previously a functional separation between ryanodine- and nonryanodine-sensitive stores, we determined next whether cytosolic Ca2⫹ elevation evoked by ESS stimuli depends on Ca2⫹ release from nonryanodine (IP3-sensitive) stores. For this purpose, we evaluated the presence and characteristics of ESS responses after the depletion of ryanodine-sensitive stores. As with BK responses, no major changes in the Ca2⫹ signals elicited by ESS ligands were observed following the depletion of ryanodine-sensitive stores. The depletion of ryanodine stores only affected ESS signals in the cell body region of VN neurons (Fig. 10B, a). Consistent with similar effects observed in Ca2⫹ transients elicited by K⫹ depolarization (Fig. 4C) and BK stimuli (Fig. 8B), it is likely that these effects depend on an impairment in CICR mechanisms that we have interpreted previously to be mediated predominantly by Ca2⫹ release from ryanodine-sensitive pools. CHEMOATTRACTANT-INDUCED CALCIUM RELEASE 1465 interfere with IP3 mechanisms (Worley et al. 1988) and ESS responses seem to rely on this pathway, we also tested the effect of blocking the exchanger by choline substitution (n ⫽ 3). Consistent with our previous results, the replacement of Na⫹ by choline yielded similar effects to those obtained using Li⫹. As illustrated in Fig. 12C, under this condition, ESS responses also exhibited larger amplitudes and longer durations, and these changes resulted mainly from the slower decay rate of the cytosolic Ca2⫹ elevations elicited by ESS ligands. Thus both Li⫹ and choline substitution confirm that during VN transduction the Na⫹/ Ca2⫹ exchanger participates in the clearance of Ca2⫹ overloads. DISCUSSION General characteristics of optical signals in snake VN neurons In this study we have shown the presence of major cytosolic Ca2⫹ transients associated with chemosensory transduction in the J Neurophysiol • VOL VNO of the garter snake. The combined use of optical techniques with the selective labeling of VN neurons by retrograde transport of the Ca2⫹ indicator made it possible for us to assess the characteristics and sources of cytosolic Ca2⫹ elevations elicited by a well-defined chemoattractant exclusively in VN neuron. In our slice preparation, the relatively low resolution of the images in relation to the image blur caused by the lack of confocal optics (Cinelli 2000) prevented single-cell resolution of fluorescence signals in most cases. Nevertheless, two important experimental conditions have facilitated the analysis of Ca2⫹ responses in snake VN neurons. First, fluorescence signals in this study arose exclusively from VN neurons because only these cells were retrogradely labeled with the Ca2⫹ indicator from the AOB. Therefore no optical signals could arise from cell types other than mature VN neurons whose axons project to the AOB. Another advantage in the snake VNO is its laminar organization. As illustrated in Fig. 1B, the cell bodies of mature VN neurons are located in the 87 • MARCH 2002 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 2⫹ FIG. 11. Contribution of the cAMP signaling pathway to the generation of ESS-elicited Ca transients. Cyclic nucleotide-gated (CNG) channel blockers evoked no detectable effects either in the spatial distribution nor the time courses of Ca2⫹ signals evoked by ESS. Top: images show the spatial distribution of Ca2⫹ signals elicited by ESS ligands (2.2 mg/ml) under control conditions (A) and after preincubation of the VN slices (10 min) with the CNG channel blockers LY83583 (40 M; B) and L-cis-diltiazem (LCD, 20 M; C). Patterns correspond to the amplitude peaks of the Ca2⫹ responses plots illustrated in D and E. No detectable changes were observed either in the spatial distribution or the time course of ESS responses following incubation with CNG blockers. F: plot corresponds to cytosolic Ca2⫹ levels during the application of forskolin (5 M) to the bath; no measurable changes in [Ca2⫹]i were observed. Optical signals were obtained from single runs (1 frame/s; 120-ms image exposure). The 1st point in the plots corresponds to basal cytosolic values and the 2nd point to the 1st response obtained 1 s after stimulus application. Cytosolic Ca2⫹ levels were coded in pseudocolors as in Fig. 1C. Abbreviations as in Fig. 1. Horizontal bar in C ⫽ 40 m. 1466 A. R. CINELLI, D. WANG, P. CHEN, W. LIU, AND M. HALPERN intermediate region of the sensory epithelium with dendrites projecting apically, toward the epithelial lumen. Thus optical signals recorded at intermediate epithelial levels represented Ca2⫹ changes in the cell body regions, while signals toward the luminal epithelial surface corresponded to Ca2⫹ responses from VN dendrites. In general we found that the average basal [Ca2⫹]i values in snake VN neurons were in reasonable agreement with those found in other neurons, including ORNs. The cytosolic Ca2⫹ elevations elicited by the chemoattractant ESS also exhibited characteristics and kinetics similar to the Ca2⫹ responses evoked in rat VN neurons (Leinders-Zufall et al. 2000) and ORNs (Leinders-Zufall et al. 1997, 1998; Restrepo and Boyle 1991; Restrepo et al. 1990, 1993; Sato et al. 1991; Tareilus et al. 1995) during chemosensory transduction. J Neurophysiol • VOL Spatial distribution of Ca2⫹ transient elicited by ESS ligands An interesting finding was that Ca2⫹ signals elicited by ESS ligands in snake VN neurons exhibited a rather broad distribution with optical signals arising from an apparently large population of VN neurons. These Ca2⫹ transients, however, were not uniformly distributed and exhibited dissimilar thresholds, characteristics that suggest the presence of heterogeneous responses in different VN neurons. We also observed a recruitment of new VN neurons that became active as ESS concentration increased. But even at the highest concentration tested not all VN neurons responded to ESS stimuli, suggesting a certain degree of selectivity in the response characteristics of these neurons. Corroborating this notion, the heterogeneous response characteristics among different VN neurons con- 87 • MARCH 2002 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 ⫹ 2⫹ FIG. 12. Contribution of the Na /Ca exchanger in the generation of ESS-elicited Ca2⫹ transients. Left: images and the corresponding time-course plots (right) show the spatial distribution of ESS-elicited Ca2⫹ signals in normal Ringer solution (A) and after replacing Na⫹ with Li⫹ (B) or with choline (C) in the bath to block Na⫹/Ca2⫹ exchanger activity. Images correspond to frames taken during the peak and late phases of the responses (frames 2nd and 8th, respectively). Under both conditions that block Na⫹/Ca2⫹ exchanger activity, there was a significant increase in response magnitude during the late phases of these responses that was also accompanied by a slight increase in peak magnitude. Optical signals were obtained from single runs (1 frame/s; 120-ms image exposure). The 1st point in the plots corresponds to basal cytosolic values and the 2nd point to the 1st response obtained 1 s after stimulus application. Cytosolic Ca2⫹ levels were coded in pseudocolors as in Fig. 1. Abbreviations as in Fig. 1. CHEMOATTRACTANT-INDUCED CALCIUM RELEASE Mechanisms involved in Ca2⫹ transients in VN neurons Our results indicate that in snake VN neurons, Ca2⫹ responses elicited by ESS ligands depend on two different mechanisms: a Ca2⫹ influx through the plasma membrane and a Ca2⫹ release from intracellular stores. We can assume that these two components correspond to cytosolic Ca2⫹ elevations exclusively from VN neurons because only these cellular elements were stained and contribute to the optical response evaluated here (see preceding text). The component generated by a Ca2⫹ influx was found after depleting intracellular stores with thapsigargin and exclusively observed in the apical dendritic region of VN neurons. This distribution is interesting because it suggests that this component occurs at locations in close proximity to the sites where VN transduction takes place and could be related to the activation of a primary transduction current. The component dependent on Ca2⫹ release from internal stores displayed a more widespread distribution with responses spreading to different cellular segments. Nevertheless, it seems likely that this component also starts in apical dendritic regions because there it exhibited its lowest threshold. If this is the case, it is interesting that both Ca2⫹ influx and Ca2⫹ release seem to coexist in similar dendritic domains. Within the limitation imposed by the time resolution of our recordings, both components seem to appear almost simultaJ Neurophysiol • VOL neously because we were unable to detect any difference either in their latencies or their rise times. It is unlikely that this characteristic results from the “in vitro” conditions used here because ESS ligands should only stimulate VN neurons through the activation of VN receptors. Thus even in our slice preparation, only responses associated with VN transduction should give rise to these Ca2⫹ signals. We also found these two components to be relatively independent because the suppression of one of them did not abolish the other. In fact, the depletion of internal stores by thapsigargin abolishes most Ca2⫹ transients elicited by ESS ligands except those arising from a Ca2⫹ influx. On the other hand, this Ca2⫹ influx was reversibly abolished when Ca2⫹ was removed from the medium, but this condition did not suppress the Ca2⫹ signals elicited from Ca2⫹ release. Therefore we cannot consider either of these two components to be secondary to the other. Instead it is likely that both components constitute primary responses that appear to be triggered in parallel by a common mechanism associated with the initial stages of VN signal transduction. BK applications evoked Ca2⫹ transients that exhibited kinetics and properties similar to the Ca2⫹ signals evoked by ESS ligands. The main difference observed was in relation to their spatial distribution. In contrast to the heterogeneous appearance of ESS responses, Ca2⫹ signals elicited by BK stimuli were distributed more uniformly among different VN neurons. This characteristic probably reflects the lack of selectivity of the BK stimulus in activating different VN neurons. But similar to ESS responses, Ca2⫹ signals elicited by BK also exhibited two components, one arising from Ca2⫹ influx and the other from Ca2⫹ release. The component arising from Ca2⫹ influx was also found exclusively in apical dendritic regions close to the epithelial lumen. This is an interesting finding because in our “in vitro” slice preparation, BK stimuli directly applied to the bath probably activate BK receptors from all cellular segments because it is believed that in general these receptors are broadly distributed in neurons (Koizumi et al. 1999). Despite this putative global effect, however, we observed in VN neurons only a Ca2⫹ influx in apical dendritic segments, indicating that this component probably exists only at this cellular level. This evidence provides further support for the notion that it might represent the opening of a conductance that is normally activated by VN transduction. In addition, as with ESS signals, these two components also appear to be relatively independent because the suppression of one did not abolish the presence of the other. Finally, Ca2⫹ release in response to both ESS and BK was largely unaffected by the selective depletion of ryanodine-sensitive stores (see following text). This finding indicates that this Ca2⫹ release depends predominantly on nonryanodine-sensitive stores, probably those stores activated by IP3 (Berridge 1998). Only the Ca2⫹ responses from the cell body seem to be affected by the depletion of ryanodine stores but with reductions that appear to be secondary because only the late phases of these Ca2⫹ transients are affected. Taken together with the evidence obtained in the biochemical assay indicating that both ESS and BK stimuli evoked similar IP3 elevations, these results lead to the interpretation that the Ca2⫹ transients elicited by ESS ligands depend primarily on mechanisms linked to IP3 production. Consistent with this interpretation, previous functional studies have sug- 87 • MARCH 2002 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 trasted with the more uniform signals evoked by other stimuli such as caffeine, high-[K⫹]o depolarization and BK. The broad and heterogeneous responses elicited by ESS stimuli in snake VN neurons contrast with the more restricted patterns found in mouse VN neurons following stimulation with single chemicals (Leinders-Zufall et al. 2000). In this case, Ca2⫹ transients were found only in limited VN neurons, the number of which remained constant at higher stimulus concentrations. It is likely that the differences observed in the distribution of Ca2⫹ signals in these two preparations may arise as a consequence of the different types of stimuli employed. Natural compounds such as ESS ligands have a heterogeneous molecular structure that probably comprises multiple chemical cues. Thus it is likely that each of these cues could activate different sets of VN neurons having distinct molecular receptive ranges. In contrast, pure single chemicals such as those tested in the mouse VNO probably activate a more selective and uniform set of VN neurons. Supporting this hypothesis, recent electrophysiological studies in rat VNO have shown that complex pheromonal stimulants found in urine elicit broad activation of large numbers of VN neurons (Holy et al. 2000). Moreover, similar to our findings in snake VN neurons, higher concentrations of this natural stimulus also seem to increase the number of active VN neurons. In general, in both systems it is conceivable that the simultaneous detection of multiple cues by a large number of VN neurons may reflect a distributed coding strategy that is useful for the detection of complex chemoattractants. In the garter snake, such a strategy would be useful in identifying a large number of chemical cues associated with different types of prey. A similar combinatorial code strategy has been proposed as the basis for odor recognition in the main olfactory system (Cinelli et al. 1995; Friedrich and Korsching 1997; Rubin and Katz 1999) and certain complex pheromonal compounds in invertebrates (for review, see Sorensen et al. 1998). 1467 1468 A. R. CINELLI, D. WANG, P. CHEN, W. LIU, AND M. HALPERN FIG. 13. Summary of the different mechanisms postulated to participate in the generation of Ca2⫹ transients during chemosensory transduction in snake VN neurons. Our results support the existence of 2 levels of organization in the generation of ligand-induced Ca2⫹ signals. First, ESS activates selective receptors that appear to trigger IP3 production and mediate an increase in cytosolic Ca2⫹ levels through 2 different mechanisms: a Ca2⫹ release from internal stores, and a Ca2⫹ influx through a putative IP3-gated conductance in the plasma membrane. These initial Ca2⫹ elevations appear to be activated in parallel and subsequently amplified through ⱖ2 other secondary mechanisms. One includes a further Ca2⫹ release through a Ca2⫹-induced Ca2⫹ release mechanism mediated by functionally independent ryanodine-sensitive pools. The other is dependent on Ca2⫹ influx through the activation of voltagesensitive Ca2⫹ channels following membrane depolarization. The 1st stage of these Ca2⫹ transients occurs predominantly in dendritic regions, while secondary events occur predominantly in the somata region. During these responses, Na⫹/Ca2⫹ exchanger activity contributes in limiting the magnitude and duration of these cytosolic Ca2⫹ elevations by Ca2⫹ efflux. J Neurophysiol • VOL results seem to reveal two levels of organization in the generation of ligand-induced Ca2⫹ signals. First, the binding of specific ligands to receptors triggers the activation of a secondary signaling mechanism in which IP3 production appears to be involved. This signaling molecule, in turn, appears to mediate both an increase in cytosolic Ca2⫹ levels through a release from internal stores and a Ca2⫹ influx through the plasma membrane. These initial Ca2⫹ elevations are then amplified through secondary mechanisms localized predominantly in the cell body region. These mechanisms probably include a Ca2⫹ influx through VSCC activated by membrane depolarization associated with transduction mechanisms and a further Ca2⫹ release from ryanodine-sensitive internal pools through a CICR mechanism. Thus the first stage of these Ca2⫹ transients occurs predominantly in dendritic regions, whereas secondary events occur predominantly in the somata region. Finally, the Na⫹/Ca2⫹ exchanger participates in maintaining basal cytosolic Ca2⫹ by extruding the Ca2⫹ excess resulting from activation of chemosensory transduction mechanisms. In accordance with our results, this model does not include the participation of a cAMP activation of CNG channels in the generation of the Ca2⫹ transients associated with VN transduction. Both high [K⫹]o and caffeine evoked Ca2⫹ transients near the cell body region of VN neuron, but there were important differences between these Ca2⫹ signals. High [K⫹]o elicited relatively brief Ca2⫹ signals that depended on the activation of VSCC because they were suppressed by the removal of Ca2⫹ from the medium and by common VSCC blockers. Caffeine also evoked major cytosolic Ca2⫹ elevations in the somata region, but these signals exhibited a more prolonged time course and depended exclusively on Ca2⫹ release from internal stores. These caffeine responses also differed from ESS signals at least in three aspects. First, Ca2⫹ transients evoked by caffeine exhibited a more gradual rise-time with a progressive buildup. Second, caffeine signals were absent in apical dendritic regions, and the removal of extracellular Ca2⫹ did not reduce the magnitude of these responses in any region. Third, Ca2⫹ signals elicited by caffeine were completely abolished by depletion of ryanodine-sensitive stores, indicating that these responses arise entirely from Ca2⫹ release from these pools. An interesting finding of this study was that depletion of ryanodine stores did not affect the overall characteristics of ESS responses. These data indicate that a Ca2⫹ release from ryanodine-sensitive stores does not constitute the primary source of the Ca2⫹ elevations associated with chemosensory transduction. In addition, it suggests that in snake VN neurons a functional separation exists between IP3 and ryanodinesensitive Ca2⫹ pools, which appear to be unevenly distributed among different cellular segments. In this regard, we observed that caffeine responses were found predominantly in the cell body region, while ESS and BK responses were more broadly distributed toward dendritic regions. Our data also suggest that ryanodine-sensitive pools mediate an amplification of Ca2⫹ responses through a CICR mechanism because depletion of these stores affected the decay phase of the Ca2⫹ transients evoked either by ESS or high-[K⫹]o depolarization. In rat ORNs, a similar CICR mechanism also seems to amplify Ca2⫹ signals elicited by odor stimuli (Zufall et al. 2000). 87 • MARCH 2002 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 gests that IP3 is a good candidate as a second messenger in VN transduction. In the garter snake VN epithelium, exposure of prey-derived chemoattractants induced dose-dependent IP3 accumulation (Luo et al. 1994), and similar IP3 elevations have been found in microvillar membranes from prepubertal female porcine VNO incubated with boar seminal fluid or urine (Wekesa and Anholt 1997). Aphrodisin, which is a pheromone excreted by female hamsters, also induces IP3 accumulation in the VN epithelium of male hamsters (Kroner et al. 1996). Finally, urinary pheromones evoke IP3 increases in rat VN epithelium (Sasaki et al. 1999), and the VN neuronal discharges evoked by these urinary pheromones are selectively blocked by the PLC inhibitor U-73122 (Holy et al. 2000). Besides the putative IP3-mediated Ca2⫹ responses, we also found that other mechanisms contribute to the generation of cytosolic Ca2⫹ elevations elicited by chemoattractants. Figure 13 is a summary diagram of the mechanisms that might participate in the generation and regulation of Ca2⫹ transients in VN neurons during chemosensory transduction. Basically, our CHEMOATTRACTANT-INDUCED CALCIUM RELEASE Component of ESS response dependent on a Ca2⫹ release Component of ESS response dependent on Ca2⫹ influx Besides the contribution of Ca2⫹ release, we found, in the region adjacent to the luminal surface of the VN epithelium, a component of ESS responses that depends on Ca2⫹ influx. In our recordings, this component only represents 12–20% of the total cytosolic Ca2⫹ transients elicited by ESS ligands, but the magnitude could be underestimated, since our optical signals represent population responses from several VN neurons together. Under these conditions, stronger neighboring signals arising from Ca2⫹ release might minimize the real magnitude of this Ca2⫹ influx, which probably occurs in confined regions of the microvilli. It is interesting that this Ca2⫹ influx does not appear to be a cAMP-mediated mechanism because specific blockers of CNG channels do not affect, to any measurable degree, the characteristics of this component, nor does the direct application of forskolin evoke detectable [Ca2⫹]i changes. This interpretation contrasts with the primary mechanism generating Ca2⫹ responses in ORNs (Leinders-Zufall et al. 1997, 1998) and leads to the suggestion that in snake VN neurons a cAMP signaling pathway does not play a fundamental role during the initial stages of VN signal transduction. This Ca2⫹ influx does not depend either on the reverse operation of the Na⫹/Ca2⫹ exchanger (DiPolo and Beauge 1987) because we found that its blockage resulted in an increase in the amplitude and duration of the Ca2⫹ transients. Thus this exchanger probably participates in restoring basal [Ca2⫹]i as has been reported for other neurons (Fierro et al. 1998), including ORNs (Jung et al. 1994; Noe et al. 1997; Reisert and Matthews 1998; Zufall et al. J Neurophysiol • VOL 2000). Because we found that both ESS and BK responses seem to depend on IP3 production, it appears likely that this Ca2⫹ influx depends on activation of an IP3 conductance in VN dendritic membranes. The existence of IP3-gated cation channels has been proposed in different chemosensory systems, but unfortunately their role is still unclear (for review, see Schild and Restrepo 1998). The best available evidence for the existence of these types of channels in chemosensory transduction is in lobster ORNs, where it has been possible to characterize an IP3-gated channel coupled to a G protein (Munger et al. 2000). In vertebrates, however, the presence of an IP3 transduction pathway in olfaction is still controversial. Transgenic mice deficient in either CNG channels or G-protein-coupled adenylate cyclase type III (Golf) fail to respond to odors (Belluscio et al. 1999; Brunet et al. 1996), suggesting that IP3 cannot be considered an alternate transduction pathway in this system. Among the possible candidates involved in mediating the Ca2⫹ influx found here are members of the transient receptor potential (TRP) channels. Some TRP proteins are nonspecific cation-permeable, store-operated channels (SOC) activated by the depletion of intracellular Ca2⫹ stores (Boulay et al. 1999; Minke and Selinger 1996a,b; Vannier et al. 1999; Zhu et al. 1996). However, it has been reported that some members in this family seem to be activated also by phosphoinositide turnover but not after store depletion (Okada et al. 1998; Schaefer et al. 2000). This may involve both functional and physical interactions between IP3 receptors and TRP channels (Birnbaumer et al. 2000; Boulay et al. 1999; Kiselyov et al. 1998). In situ hybridization and immunohistochemical studies (Liman et al. 1999) in rats indicate that the mRNA of a member of this channel type (rTRP2) is expressed exclusively in the receptor cells of the vomeronasal sensory epithelium and that the protein is localized to the microvilli of these neurons. Interestingly, this location corresponds to the Ca2⫹ influx here. If similar TRP channels are expressed in snake VN neurons in this region, it is unlikely that they would be solely SOC because our results indicate that depletion of intracellular Ca2⫹ stores alone failed to elicit the Ca2⫹ influx observed in this study. Still to be resolved are the relationship between these two components found in responses to ESS and BK as well as the exact role of these cytosolic Ca2⫹ elevations during VN transduction. It is likely, however, that these cytosolic Ca2⫹ elevations play a significant role in the regulation of VN responses. In the main olfactory system, cytosolic Ca2⫹ elevations with similar kinetics play an important role, among other functions, in odor adaptation. In ORNs, the main mechanism of odor adaptation depends on an increase in cytosolic Ca2⫹ that activates a calmodulin (CAM)-dependent protein kinase II (CAMkinase II) that, in turn, phosphorylates the adenyl cyclase and lowers CNG channel activity (Chen and Yau 1994; Kurahashi and Menini 1997). A similar mechanism is unlikely to occur in snake VN neurons because we found that Ca2⫹ transients associated with VN transduction do not depend on the activation of CNG channels. Other mechanisms, however, can still be triggered by cytosolic Ca2⫹ and act on either the primary transduction currents or downstream. Interestingly, in Caernorhabditis elegans (Colbert et al. 1997) and Drosophila (Störtkuhl et al. 1999) mutants defective in some TRP members exhibit impairment in olfactory adaptation. In addition, 87 • MARCH 2002 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 An unexpected finding of this study was the presence of major Ca2⫹ transients elicited by ESS ligands in the absence of extracellular Ca2⫹. This result differs from otherwise similar Ca2⫹ signals associated with chemosensory transduction in mouse VN neurons (Leinders-Zufall et al. 2000) and in ORNs (Leinders-Zufall et al. 1997; Restrepo et al. 1990, 1993; Sato et al. 1991; Schild et al. 1995; Tareilus et al. 1995), which are completely suppressed in 0 [Ca2⫹]o. It is interesting to note that Ca2⫹ release from internal Ca2⫹ pools also occurs in ORNs, but this mechanism appears to be secondary to an initial Ca2⫹ influx that is mediated through the opening CNG channels (Leinders-Zufall et al. 1998; Zufall et al. 2000). In snake VN neurons, we found that Ca2⫹ release can be directly triggered by VN transduction mechanisms, a condition that may depend on two factors. First, in this system transduction seems to be mediated by phosphoinositide turnover, and it is known that IP3 elevations in multiple cell systems mediate major Ca2⫹ mobilization from intracellular pools (for review, see Berridge 1993, 1998). Second, in snake VN neurons, it appears that a tight coupling exists between the IP3 elevation associated with VN signal transduction and the activation of Ca2⫹ release from intracellular stores. Previous ultrastructural studies of these cells demonstrated well developed cisternae of endoplasmic reticulum (ER) extending close to microvillar processes (Wang and Halpern 1980a,b). Perhaps, the proximity of these potential Ca2⫹ stores to the microvilli permits efficient coupling for the activation of Ca2⫹ release during VN signal transduction. 1469 1470 A. R. CINELLI, D. WANG, P. CHEN, W. LIU, AND M. HALPERN This work was supported by National Institute on Deafness and Other Communication Disorders Grant DC-02531 (M. Halpern) and National Science Foundation Grant IBN9905700 (D. Wang). REFERENCES BELLUSCIO L, KOENTGES G, AXEL R, AND DULAC C. A map of pheromone receptor activation in the mammalian brain. Cell 97: 209 –220, 1999. BERRIDGE MJ. Inositol trisphosphate and Ca2⫹ signalling. Nature 361: 315– 325, 1993. BERRIDGE MJ. Neuronal calcium signaling. Neuron 21: 31–36, 1998. BIRNBAUMER L, BOULAY G, BROWN D, JIANG M, DIETRICH A, MIKOSHIBA K, ZHU X, AND QIN N. Mechanism of capacitative Ca2⫹ entry (CCE): interaction between IP3 receptor and TRP links the internal calcium storage compartment to plasma membrane CCE channels. Recent Prog Horm Res 55: 127–161, 2000. BOULAY G, BROWN DM, QIN N, JIANG M, DIETRICH A, ZHU MX, CHEN Z, BIRNBAUMER M, MIKOSHIBA K, AND BIRNBAUMER L. Modulation of Ca2⫹ entry by polypeptides of the inositol 1,4,5-trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca2⫹ entry. Proc Natl Acad Sci USA 96: 14955–14960, 1999. BRUNET LJ, GOLD GH, AND NGAI J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron 17: 681– 693, 1996. BURGHARDT GM AND PRUITT GH. The role of the tongue and senses in feeding of naive and experienced garter snakes. Physiol Behav 14: 185–194, 1975. CHEN TY AND YAU KW. Direct modulation by calcium-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature 368: 545–548, 1994. CINELLI AR. Flexible, low cost, high sensitivity analog video camera. J Neurosci Methods 83: 205–216, 1998. CINELLI AR. High-definition mapping of neural activity using voltage-sensitive dyes. Methods 21: 349 –372, 2000. J Neurophysiol • VOL CINELLI AR, NEFF SR, AND KAUER JS. Neuronal activity in the olfactory bulb observed by video-rate imaging of voltage-sensitive dye fluorescence. I. Methods and characteristics of the signals. J Neurophysiol 73: 2017–2032, 1995. CINELLI AR AND SALZBERG BM. Multiple site optical recording of transmembrane voltage (MSORTV), single-unit recordings, and evoked field potentials from the olfactory bulb of skate (R. erinacea). J Neurophysiol 64: 1767–1790, 1991. CINELLI AR AND SALZBERG BM. Dendritic origin of late events in optical recordings from the salamander olfactory bulb. J Neurophysiol 68: 786 – 806, 1992. CLAPHAM DE. Calcium signalling. Cell 80: 259 –268, 1995. COLBERT HA, SMITH TL, AND BARGMANN CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci 17: 8259 – 8269, 1997. CONGAR P, LEINEKUGEL X, BEN-ARI Y, AND CREPEL VA. Long-lasting calciumactivated nonselective cationic current is generated by synaptic stimulation or exogenous activation of group I metabotropic glutamate receptors in CA1 pyramidal neurons. J Neurosci 17: 5366 –5379, 1997. DANACEAU JP AND LUCERO MT. Electrogenic Na⫹/Ca2⫹ exchange. A novel amplification step in squid olfactory transduction. J Gen Physiol 115: 759 –768, 2000. DIPOLO R AND BEAUGE L. Characterization of the reverse Na/Ca exchange in squid axons and its modulation by [Ca2⫹]i and ATP [Ca2⫹]i-dependent [Na⫹]i/[Ca2⫹]o and [Na⫹]i/[Na⫹]o exchange modes. J Gen Physiol 90: 505–525, 1987. DIPOLO R AND BEAUGE L. Metabolic pathways in the regulation of invertebrate and vertebrate Na⫹/Ca2⫹ exchange. Biochim Biophys Acta 1422: 57–71, 1999. FIERRO L, DIPOLO R, AND LLANO I. Intracellular calcium clearance in Purkinje cell somata from rat cerebellar slices. J Physiol (Lond) 510: 499 –512, 1998. FRIEDRICH RW AND KORSCHING SI. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron 18: 737–752, 1997. GRAVES BM AND DUVALL D. Avomic prairie rattlesnakes (Crotalus viridis) fail to attack rodent prey. Z Tierpsychol 67: 161–166, 1985. HALPERN M. Nasal chemical senses in snakes. In: Advances in Vertebrate Neuroethology, edited by Ewert J-P, Capranica RR, and Ingle DJ. London: Plenum, 1982, p. 141–176. HALPERN M. Nasal chemical senses in reptiles: structure and function. In: Hormones, Brain and Behavior. Biology of the Reptilia. Physiology, edited by Gans C and Crews D. Chicago, IL: University of Chicago Press, 1992, vol. 18, p. 423–523. HALPERN M AND FRUMIN N. Roles of the vomeronasal and olfactory sytems in prey attack and feeding in adult garter snakes. Physiol Behav 22: 1183– 1189, 1979. HALPERN M AND KUBIE JL. The role of the ophidian vomeronasal system in species-typical behavior. Trends Neurosci 7: 472– 477, 1984. HALPERN M, SHAPIRO LS, AND JIA C-P. Differential localization of G proteins in the opossum vomeronasal system. Brain Res 677: 157–161, 1995. HOLY T, DULAC C, AND MEISTER M. Responses of vomeronasal neurons to natural stimuli. Science 289: 1569 –1572, 2000. JIANG XC, INOUCHI J, WANG D, AND HALPERN M. Purification and characterization of a chemoattractant from electric shock-induced secretion and its receptor binding and signal transduction through vomeronasal system of garter snakes. J Biol Chem 265: 8736 – 8744, 1990. JUNG A, LISCHKA FW, ENGEL J, AND SCHILD D. Sodium/calcium exchanger in olfactory receptor neurones of Xenopus laevis. Neuroreport 5: 1741–1744, 1994. KAHMANN H. Sennesphysiologische studien an Reptilien. I. Experimentelle Untersuchunge uber das Jakobsonische Organ der Eidenschsen und Schlangen. Zool Jahrb Abt Allg Zool Physiol Tiere 51: 173–238, 1932. KIRISCHUK S, MOLLER T, VOITENKO N, KETTENMANN H, AND VERKHARATSKY A. ATP-induced cytoplasmic calcium mobilization in Bergmann glial cells. J Neurosci 15: 7861–7871, 1995. KISELYOV K, XU X, MOZHAYEVA G, KUO T, PESSAH I, MIGNERY G, ZHU X, BIRNBAUMER L, AND MUALLEM S. Functional interaction between InsP3 receptors and store-operated Htrp 3 channels. Nature 396: 478 – 482, 1998. KLEENE SJ. Origin of the chloride current in olfactory transduction. Neuron 11: 123–132, 1993. KLEENE SJ. High-gain, low-noise amplification in olfactory transduction. Biophys J 73: 1110 –1117, 1997. 87 • MARCH 2002 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 cytosolic Ca2⫹ elevations could also regulate VN responses by modulating membrane excitability as has been demonstrated in a variety of neurons (Congar et al. 1997; Llano et al. 1991; Partridge and Valenzuela 1999; Partridge et al. 1994). IP3 dialyzed into snake vomeronasal receptor cells produces a depolarizing current, which has been attributed to chloride (Taniguchi et al. 2000). According to our results, it is possible that IP3 dialysis induces a cytosolic Ca2⫹ increase that in turn could activate a Cl⫺ conductance. In this regard, Ca2⫹-dependent Cl⫺ conductance is well characterized in ORNs and constitutes an important mechanism for boosting the initial membrane depolarization caused by the opening of CNG channels (Kleene 1993, 1997; Kleene and Gesteland 1991; Kurahashi and Yau 1993). In addition cytosolic Ca2⫹ levels could also modulate the activity of other conductances such as K⫹ channels and VSCC as occurs in ORNs (for review, see Schild and Restrepo 1998). On the other hand, if TRP channels constitute the primary transduction currents in VN neurons, Ca2⫹ release occurring in or adjacent to the microvilli could regulate the activity of these channels. Although there are some members of the TRP channel family that are not store operated, still the depletion of intracellular stores could enhance their activity (Strubing et al. 2001). Under such conditions, the depletion of intracellular Ca2⫹ stores could potentiate the cation influx through these channels, a condition that could eventually enhance transduction currents. Thus the presence of cytosolic Ca2⫹ transients arising from two sources simultaneously in VN neurons is of special interest in light of the accumulating body of evidence indicating that cytosolic Ca2⫹ plays an important role in modulating sensory transduction at different levels. CHEMOATTRACTANT-INDUCED CALCIUM RELEASE J Neurophysiol • VOL RUBIN BD AND KATZ LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron 23: 499 –511, 1999. SASAKI K, OKAMOTO K, INAMURA K, TOKUMITSU Y, AND KASHIWAYANAGI M. Inositol-l,4,5-trisphosphate accumulation induced by urinary pheromones in female rat vomeronasal epithelium. Brain Res 823: 161–168, 1999. SATO T, HIRONO J, TONOIKE M, AND TAKEBAYASHI M. Two types of increases in free Ca2⫹ evoked by odor in isolated frog olfactory receptor neurons. Neuroreport 2: 229 –232, 1991. SCHAEFER M, PLANT TD, OBUKHOV AG, HOFMANN T, GUDERMANN T, AND SCHULTZ G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J Biol Chem 275: 17517–17526, 2000. SCHILD D, LISCHKA FW, AND RESTREPO D. InsP3 causes an increase in apical [Ca2⫹]i by activating two distinct current components in vertebrate olfactory receptor cells. J Neurophysiol 73: 862– 866, 1995. SCHILD D AND RESTREPO D. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol Rev 78: 429 – 466, 1998. SEYMOUR-LAURENT KJ AND BARISH ME. Inositol 1,4,5-trisphosphate and ryanodine receptor distributions and patterns of acetylcholine- and caffeineinduced calcium release in cultured mouse hippocampal neurons. J Neurosci 15: 2592–2608, 1995. SORENSEN PW, CHRISTENSEN TA, AND STACEY NE. Discrimination of pheromonal cues in fish: emerging parallels with insects. Curr Opin Neurobiol 8: 458 – 467, 1998. STÖRTKUHL KF, HOVEMANN BT, AND CARLSON JR. Olfactory adaptation depends on the Trp Ca2⫹ channel in Drosophila. J Neurosci 19: 4839 – 4846, 1999. STRUBING C, KRAPIVINSKY G, KRAPIVINSKY L, AND CLAPHAM DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29: 645– 655, 2001. TANIGUCHI M, KASHIWAYANAGI M, AND KURIHARA K. Intracellular injection of inositol 1,4,5-trisphosphate increases a conductance in membranes of turtle vomeronasal receptor neurons in the slice preparation. Neurosci Lett 188: 5– 8, 1995. TANIGUCHI M, KASHIWAYANAGI M, AND KURIHARA K. Intracellular dialysis of cyclic nucleotides induced inward currents in turtle vomeronasal receptor neurons. J Neurosci 16: 1239 –1246, 1996. TANIGUCHI M, WANG D, AND HALPERN M. The characteristics of the electrovomeronasogram (EVG): its loss following vomeronasal axotomy in garter snake. Chem Senses 23: 653– 659, 1998. TANIGUCHI M, WANG D, AND HALPERN M. Chemosensitive conductance and inositol 1,4,5-trisphosphate-induced conductance in snake vomeronasal receptor neurons. Chem Senses 25: 67–76, 2000. TAREILUS E, NOE I, AND BREER H. Calcium signals in olfactory neurons. Biochim Biophys Acta 1269: 129 –138, 1995. TSIEN RY, RINK TJ, AND POENIE M. Measurement of cytosolic free calcium in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium 6: 145–157, 1985. USACHEV Y, SHMIGAL A, PRONCHUK N, KOSTYUK P, AND VERKHRATSKY A. Caffeine-induced calcium release from internal stores in cultured rat sensory neurons. Neuroscience 57: 845– 859, 1993. USACHEV YM AND THAYER SA. Ca2⫹ influx in resting rat sensory neurones that regulates and is regulated by ryanodine-sensitive Ca2⫹ stores. J Physiol (Lond) 519: 115–130, 1999. VANNIER B, PEYTON M, BOULAY G, BROWN D, QIN N, JIANG M, ZHU X, AND BIRNBAUMER L. Mouse Trp2 the homologue of the human Trpc2 pseudogene, encodes mTrp2, a store depletion-activated capacitative Ca2⫹ entry channel. Proc Natl Acad Sci USA 96: 2060 –2064, 1999. VERKHRATSKY A AND KETTENMANN H. Calcium signalling in glial cells. Trends Neurosci 19: 346 –352, 1996. WEKESA KS AND ANHOLT RR. Pheromone regulated production of inositol(1,4,5)-trisphosphate in the mammalian vomeronasal organ. Endocrinology 138: 3497–3504, 1997. WANG D, CHEN P, AND HALPERN M. Immunological analysis of earthwormderived chemoattractive proteins. Comp Biochem Physiol B Biochem Mol Biol 110: 601– 610, 1992. WANG D, CHEN P, JIANG X-C, AND HALPERN M. Isolation from earthworms of a proteinaceous chemoattractant to garter snakes. Arch Biochem Biophys 267: 459 – 466, 1988. WANG D, CHEN P, LIU W, LI C-S, AND HALPERN M. Chemosignal transduction in the vomeronasal organ of garter snakes: Ca2⫹-dependent regulation of adenylate cyclase. Arch Biochem Biophys 348: 96 –106, 1997. WANG D, JIANG XC, CHEN P, INOUCHI J, AND HALPERN M. Chemical and immunological analysis of prey-derived vomeronasal stimulants. Brain Behav Evol 41: 246 –254, 1993. 87 • MARCH 2002 • www.jn.org Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 KLEENE SJ AND GESTELAND RC. Calcium-activated chloride conductance in frog olfactory cilia. J Neurosci 11: 3624 –3629, 1991. KOIZUMI S, BOOTMAN MD, BOBANOVI LK, SCHELL MJ, BERRIDGE MJ, AND LIPP P. Characterization of elementary Ca2⫹ release signals in NGF-differentiated PC12 cells and hippocampal neurons. Neuron 22: 125–137, 1999. KOLESNIKOV SS, ZHAINAZAROV AB, AND KOSOLAPOV AV. Cyclic nucleotideactivated channels in the frog olfactory receptor plasma membrane. FEBS Lett 266: 96 –98, 1990. KRONER C, BREER H, SINGER AG, AND O’CONNELL RJ. Pheromone-induced second-messenger signaling in the hamster vomeronasal organ. Neuroreport 7: 2989 –2992, 1996. KUBIE JL AND HALPERN M. The chemical senses involved in garter snake prey trailing. J Comp Physiol Psychol 93: 648 – 667, 1979. KURAHASHI T AND MENINI A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature 385: 725–729, 1997. KURAHASHI T AND YAU KW. Co-existence of cationic and chloride components in odorant-induced current of vertebrate olfactory receptor cells. Nature 363: 71–74, 1993. LEIDERS-ZUFALL T, GREER CA, SHEPHERD GM, AND ZUFALL F. Imaging odorinduced Ca2⫹ transients in single olfactory cilia: specificity of activation and role in transduction. J Neurosci 18: 5630 –5639, 1998. LEINDERS-ZUFALL T, LANE A, PUCHE A, MA W, NOVOTNY M, SHIPLEY M, AND ZUFALL F. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature 405: 792–796, 2000. LEINDERS-ZUFALL T, RAND MN, SHEPHERD GM, GREER CA, AND ZUFALL F. Calcium entry through cyclic nucleotide-gated channels in individual cilia of olfactory receptor cells: spatiotemporal dynamics. J Neurosci 17: 4134 – 4148, 1997. LIMAN ER, COREY DP, AND DULAC C. TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proc Natl Acad Sci USA 96: 5791–5796, 1999. LIU W, WANG D, LIU J, CHEN P, AND HALPERN M. Cloning of a gene encoding adenylate cyclase from vomeronasal organ of garter snakes. Arch Biochem Biophys 358: 204 –210, 1998. LLANO I, DREESSEN J, KANO M, AND KONNERTH A. Intradendritic release of calcium induced by glutamate in cerebellar Purkinje cells. Neuron 7: 577– 583, 1991. LUO Y, LU S, CHEN P, WANG D, AND HALPERN M. Identification of chemoattractant receptors and G proteins in the vomeronasal system of garter snakes. J Biol Chem 269: 16867–16877, 1994. MINKE B AND SELINGER Z. Role of Drosophila TRP in inositide-mediated Ca2⫹ entry. Mol Neurobiol 12: 163–180, 1996a. MINKE B AND SELINGER Z. The roles of trp and calcium in regulating photoreceptor function in Drosophila. Curr Opinion Neurobiol 6: 459 – 466, 1996b. MUNGER SD, GLEESON RA, ALDRICH HC, RUST NC, ACHE BW, AND GREENBERG RM. Characterization of a phosphoinositide-mediated odor transduction pathway reveals plasma membrane localization of an inositol 1,4,5trisphosphate receptor in lobster olfactory receptor neurons. J Biol Chem 275: 20450 –20457, 2000. NAULLEAU G. La biologie et le comportement predateur de Vipera aspis au laboratoire et dans la nature. Bull Biol France Belgique 99: 395–524, 1965. NOE J, TAREILUS E, BOEKHOFF I, AND BREER H. Sodium/calcium exchanger in rat olfactory neurons. Neurochem Int 30: 523–531, 1997. OKADA T, SHIMIZU S, WAKAMORI M, MAEDA A, KUROSAKI T, TAKADA N, IMOTO K, AND MORI Y. Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2⫹ channel from mouse brain. J Biol Chem 273: 10279 –10287, 1998. PARTRIDGE LD, MÒLLER TH, AND SWANDULLA D. Calcium-activated nonselective channels in the nervous system. Brain Res Rev 19: 319 –325, 1994. PARTRIDGE LD AND VALENZUELA CF. Ca2⫹ store-dependent potentiation of Ca2⫹-activated non-selective cation channels in rat hippocampal neurones in vitro. J Physiol (Lond) 521: 617– 627, 1999. REISERT J AND MATTHEWS HR. Na⫹-dependent Ca2⫹ extrusion governs response recovery in frog olfactory receptor cells. J Gen Physiol 112: 529 – 535, 1998. RESTREPO D AND BOYLE AG. Stimulation of olfactory receptors alters regulation of [Ca2⫹]i in olfactory neurons of the catfish (Ictalurustmnctatus). J Membr Biol 120: 223–232, 1991. RESTREPO D, MIYAMOTO T, BRYANT BP, AND TEETER JH. Odor stimuli trigger influx of calcium into olfactory neurons of the channel catfish. Science 249: 1166 –1168, 1990. RESTREPO D, OKADA Y, AND TEETER JH. Odorant-regulated Ca2⫹ gradients in rat olfactory neurons. J Gen Physiol 102: 907–924, 1993. 1471 1472 A. R. CINELLI, D. WANG, P. CHEN, W. LIU, AND M. HALPERN WANG RT AND HALPERN M. Light and electron microscopic observations on the normal structure of the vomeronasal organ of garter snakes. J Morphol 164: 47– 67, 1980a. WANG RT AND HALPERN M. Scanning electron microscopic studies of the surface morphology of the vomeronasal epithelium and olfactory epithelium of garter snakes. Am J Anat 157: 399 – 428, 1980b. WILDE WS. The role of Jacobson’s organ in the feeding reaction of the common garter snake Thamnophis sirtalis sirtalis (Linn.). J Exp Zool 77: 445– 465, 1938. WORLEY PF, HELLER WA, SNYDER SH, AND BARABAN JM. Lithium blocks a phosphoinositide-mediated cholinergic response in hippocampal slices. Science 239: 1428 –1429, 1988. ZHU X, JIANG M, PEYTON M, BOULAY G, HURST R, STEFANI E, AND BIRNBAUMER L. TRP, a novel mammalian gene family essential for agonistactivated capacitative Ca2⫹ entry. Cell 85: 661– 671, 1996. ZUFALL F, LEINDERS-ZUFALL T, AND GREER CA. Amplification of odor-induced Ca2⫹ transients by store-operated Ca2⫹ release and its role in olfactory signal transduction. J Neurophysiol 83: 501–512, 2000. Downloaded from http://jn.physiology.org/ by 10.220.33.3 on June 14, 2017 J Neurophysiol • VOL 87 • MARCH 2002 • www.jn.org