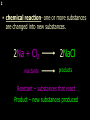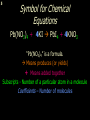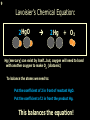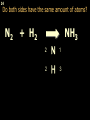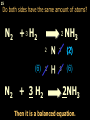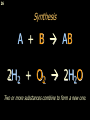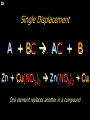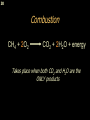* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemical Equations and Reactions
California Green Chemistry Initiative wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Asymmetric induction wikipedia , lookup
Chemical Corps wikipedia , lookup
Process chemistry wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Catalytic reforming wikipedia , lookup
Chemical plant wikipedia , lookup
Hydrogen bond wikipedia , lookup
Chemical industry wikipedia , lookup
Chemical potential wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Spinodal decomposition wikipedia , lookup
Safety data sheet wikipedia , lookup
Biochemistry wikipedia , lookup
Rate equation wikipedia , lookup
Isotopic labeling wikipedia , lookup
Marcus theory wikipedia , lookup
Click chemistry wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Chemical bond wikipedia , lookup
Chemical equilibrium wikipedia , lookup
George S. Hammond wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Electrolysis of water wikipedia , lookup
Water splitting wikipedia , lookup
Molecular dynamics wikipedia , lookup
Electrochemistry wikipedia , lookup
History of chemistry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Hydrogen atom wikipedia , lookup
Chemical reaction wikipedia , lookup
Metalloprotein wikipedia , lookup
Transition state theory wikipedia , lookup
History of molecular theory wikipedia , lookup
Stoichiometry wikipedia , lookup
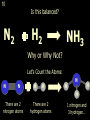
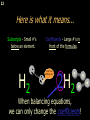
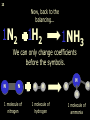
1 Chemical Equations and Reactions What are they? 2 • chemical reaction- one or more substances are changed into new substances. 2Na + Cl2 reactants 2NaCl products Reactant – substances that react Product – new substances produced 3 Evidence of Reactions • Bubbling (gives off a gas) • Precipitate-solid that falls out of solution • Temperature change • Color change 4 Chemical Equation • Expression that describes a chemical reaction using chemical formulas and other symbols Symbol Meaning (cr) Crystalline solid (l) Liquid (g) Gas (aq) Aqueous-solid dissolved in water 5 Symbol for Chemical Equations Pb(NO3)4 + 4KI PbI4 + 4KNO3 “Pb(NO3)4” is a formula. Means produces (or yields) + Means added together Subscripts - Number of a particular atom in a molecule Coefficients – Number of molecules 6 Father of Modern Chemistry 1743 - 1794 First Described the “Law of Conservation of Mass” 7 Conservation of Mass Antoine Lavoisier found that the mass of the reactants and the products are equal, even when the states of matter change. HgO Hg + O2 He started with: He ended up with: 10g of Mercury Oxide (HgO) and 9.3g Mercury… 10 g. = 0.7 + 9.3 g. ...But what happened to the O2? Matter is neither created nor destroyed. 8 • Conservation of atoms-the number of each type of atom on the reactants side of the chemical equation MUST be equal to the number of each type of atom on the products side of the equation. • Coefficient-represent the number of units of each substance taking part in the reaction • Balanced chemical equation-the same number of atoms of each element on both sides of the equation 9 Lavoisier’s Chemical Equation: 2HgO 2 Hg + O2 Hg (mercury) can exist by itself...but, oxygen will need to bond with another oxygen to make O2 (diatomic) To balance the atoms we need to: Put the coefficient of 2 in front of reactant HgO. Put the coefficient of 2 in front the product Hg. This balances the equation! 10 Is this balanced? H2 N2 NH3 Why or Why Not? Let’s Count the Atoms: N N H H H N H H There are 2 nitrogen atoms There are 2 hydrogen atoms 1 nitrogen and 3 hydrogen… 11 Atoms can only bond in certain ways… remember the criss-cross method. H2 H2 N2 N2 H2 H2 That’s why we can’t change the subscripts. N2 H2 N2 N2 N N H H 12 Here is what it means... Subscripts - Small #’s below an element. H2 Coefficients - Large #’s in front of the formulas. I can’t live without you! 2H2 When balancing equations, we can only change the coefficients! 13 Now, back to the balancing... 1H2 1N2 1NH3 We can only change coefficients before the symbols. N N H H H N H 1 molecule of nitrogen 1 molecule of hydrogen 1 molecule of ammonia H 14 Do both sides have the same amount of atoms? N2 + H 2 NH3 2 N 1 2 H 3 15 Do both sides have the same amount of atoms? N2 + 3 H 2 (6) N 2 + 3 H2 2 NH3 2 N 1 (2) 2 H 3 (6) 2NH3 Then it is a balanced equation. 16 Four Steps to Balance Equations: 1. List the metals, nonmetals, oxygen, and hydrogen below arrow. 2. Count the number of atoms you have on both sides. 3. Balance by changing the coefficients and recounting. 4. Start the process again if it still does not balance. 17 1. Set up your equation. List the elements in this order below the equation: Metals, Nonmetals, Oxygen, and Hydrogen H2 + O2 H2O Metals Nonmetals Oxygen Hydrogen O H 18 2. Count the number of atoms you have of each on both sides. H2 + O2 H2O 2 O 1 2 H 2 19 3. Balance by changing the coefficients and recounting. 2 H2 + O2 2H2O 2 O 1 (2) (4) 2 H 2 (4) How are you going to make “H” add up to 4? Is this balanced? Yes! Need to have at least 2 “O” But it changes the number of “H” 20 Let’s try another: Mg + 2 HCl H2 + MgCl2 Need to have at least 2 “Cl” 1 Mg 1 (2) 1 Cl 2 Changing the Cl changes the “H”? (2) 1 H 2 Is this balanced? Yes! 21 Let’s try another: Na +2 HCl NaCl + H2 1 Na 1 In this case, we will start with 1 Cl 1 hydrogen since it is the only one H (2) 1 2 unbalanced. 22 But, changing the hydrogen in HCl affects the number of chlorine atoms. Na + 2 HCl 2 NaCl + H2 1 Na 1 (2) 1 Cl 1 (2) (2) 1 H 2 23 Changing the chlorine on the product side affects the sodium (Na) on the reactants side. So we must now change sodium as well. 2 Na + 2HCl 2NaCl + H2 (2) 1 Na 1 (2) (2) 1 Cl 1 (2) (2) 1 H 2 24 Independent Practice 25 5 Types of Chemical Reactions Synthesis Decomposition Single Displacement Double Displacement Combustion 26 Synthesis A + B AB 2H2 + O2 2H2O Two or more substances combine to form a new one. 27 Decomposition AB A + B 2MgO 2Mg + O2 One substance breaks down or decomposes into 2 or more simpler substances. Most reactions require heat, light or electricity. 28 Single Displacement A + BC AC + B Zn + Cu(NO3)2 Zn(NO3)2 + Cu One element replaces another in a compound 29 Double Displacement AB + CD AD + CB HCl + NaOH HOH + NaCl (H2O) The negative ion of one compound replaces the negative ion of the other compound to form 2 new compounds. Usually forms a precipitate, water or a gas. 30 Combustion CH4 + 2O2 CO2 + 2H2O + energy Takes place when both CO2 and H2O are the ONLY products 31 Independent Practice 32 Energy in Reactions • In a chemical reaction energy is either released or absorbed. – The energy can be • Heat • Light • Sound • Electricity 33 Energy in Reactions • Exothermic reactions – Some form of energy is given off by the reaction • Heat given off causes reaction mixture to feel hot • Examples-burning wood, dynamite explosion • Endothermic reactions – Energy must be provided for the reaction to take place • Absorbs so much heat that the container feels cold • Example-frequently used to obtain a metal from its ore, using an electric current, chemical cold packs