* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemical Reactions
Chemical weapon proliferation wikipedia , lookup
Biochemistry wikipedia , lookup
Chemical weapon wikipedia , lookup
Chemical potential wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Chemical Corps wikipedia , lookup
Marcus theory wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Drug discovery wikipedia , lookup
Organic chemistry wikipedia , lookup
Chemical plant wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Safety data sheet wikipedia , lookup
Asymmetric induction wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Chemical bond wikipedia , lookup
Catalytic reforming wikipedia , lookup
Chemical industry wikipedia , lookup
Isotopic labeling wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Rate equation wikipedia , lookup
Process chemistry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Molecular dynamics wikipedia , lookup
Chemical equilibrium wikipedia , lookup
History of chemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Electrochemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Water splitting wikipedia , lookup
George S. Hammond wikipedia , lookup
Click chemistry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Transition state theory wikipedia , lookup
History of molecular theory wikipedia , lookup
Chemical reaction wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
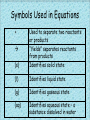
Chemical Reactions What is a chemical reaction? • A chemical reaction is the process by which the atoms of one or more substances are rearranged to form different substances. Chemical Reaction Indicators • Some ways to tell that a chemical reaction have occurred include: 1. Color change – a change indicates the particles have changed. 2. Heat content change – did the temperature go up or down. 3. Gas is produced – if it bubbles (even without being heated) it’s a gas! 4. Precipitate forms – a precipitate is a solid produced during a chemical reaction between solutions. Chemical Equations • Chemical equations are used to represent chemical reactions. 2Fe (s) + 3Cl2 (g) Reactants 2FeCl3 (s) Products Symbols Used in Equations + (s) Used to separate two reactants or products “Yields” separates reactants from products Identifies solid state (l) Identifies liquid state (g) Identifies gaseous state (aq) Identifies aqueous state – a substance dissolved in water Balanced equations • Chemical equations MUST be balanced to show that the number of atoms in the reactants is the same as the number in the products. What goes in MUST come out!!! Rules for Balancing • The only place you can change any number is the coefficient. • A coefficient is a number written in front of a chemical formula. • Don’t forget diatomic molecules. • Use the smallest ratio of coefficients possible. How to Balance • If you are starting with words, write the equation using formulas. Example: hydrogen and oxygen gases react to form water. (Hint: diatomics!) H2 + O2 H2O H2 + O2 H2O 2 hydrogens + 2 oxygens 2 hydrogens 1 oxygen Notice that there are two hydrogen atoms on each side however there are two oxygen atoms in the reactants but only one in the products. To balance this we must insert a coefficient. H2 + O2 2 H2O While that evens the number of oxygen atoms – there are now four hydrogen atoms in the products. To balance the hydrogen we go back to the reactants and insert a coefficient. 2H2 + O2 2H2O Now there are equal number of atoms of hydrogen and oxygen on each side of the equation – it is now balanced. Types of Reactions Synthesis or Combination • Synthesis is a reaction in which two or more substances react to produce a single product. A + 2Na + B AB Cl2 2NaCl Decomposition Reactions • Decomposition reactions occur when a single compound breaks down into two or more simpler substances. AB A + B or ABC A + BC 2H2O2 2H2O + O2 Mg(ClO3)2 MgCl2 + 3O2 Single-Replacement Reactions • A reaction in which the atoms of one element replace the atoms of another element in a compound. A + BC AC + B Cu + 2AgNO3 Cu(NO3)2 + 2Ag Double-Replacement Reactions • A reaction involving the exchange of positive ions between two ionic compounds dissolved in water. AB + CD AD + CB 2NaOH + CuCl2 2NaCl + Cu(OH)2 Combustion Reactions • In a combustion reaction, oxygen combines with a substance and releases energy in the form of heat and light. CH4 + 2O2 CO2 + 2H2O *All hydrocarbons contain carbon and hydrogen and burn in oxygen to yield the same products – CO2 and H2O