* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Alcohols, acids and esters
Survey
Document related concepts
Transcript
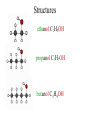
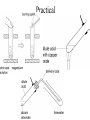
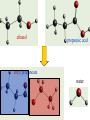
Complete the table Name Formula Structure + O2 Miscibility in water methane CH4 CO2 + H2O Immiscible ethane C2H6 CO2 + H2O Immiscible propane C3H8 CO2 + H2O Immiscible butane C4H10 CO2 + H2O Immiscible Alcohols • • • • What does the word alcohol mean to you? Like the alkanes the alcohols are a series of related compounds They all contain an –OH group – a functional group The –OH group allows them to ‘hydrogen bond’ like water does – so boiling points are higher than for the equivalent alkane Structures ethanol C2H5OH propanol C3H7OH butanol C4H9OH Reactions • + oxygen: burn to give CO2 and H2O C2H5OH (l) + 3 O2 (g) 2 CO2 (g) + 3 H2O (l) • + water: miscible • + sodium: reacts slowly producing hydrogen gas and sodium ethoxide 2 C2H5OH + 2 Na 2 C2H5O-Na+ + H2 c.f. 2 Na + 2 H2O 2 NaOH + H2 Learning objective: 28/04/2017 • To illustrate the practical importance of carboxylic acids • To demonstrate that carboxylic acids in solution show the characteristic reactions of acids • To introduce the –COOH functional group in carboxylic acids • To practise writing balanced equations to describe the reactions of carboxylic acids • To introduce esters as products of the reactions of alcohols with carboxylic acids • To observe properties of esters Carboxylic acids • Include the –COOH functional group • -C=O O H • Dissolves in water to form: e.g. CH3COOH CH3COO- + H+ • Carboxylic acids react in the same way as acids – but because they are only partially ionised at any one time they react more slowly – the concentration of [H+] is less than with, for example, HCl. They are weak acids Practical Test Observations Notes Smell e.g. vinegar Shows that there are volatile molecules i.e. does not separate fully into ions as HCl (aq) does. Weakly acidic 3-4 pH of a solution of the carboxylic acid solution 2 HCOOH (aq) + Mg (s) Reaction with Slow fizzing magnesium Produces hydrogen (HCOO)2Mg (aq) + H2 (g) metal gas & salt Reaction with Produces coloured 2 CH3COOH (aq) + CuO (s) copper oxide salt (CH3COO)2Cu (aq) + H2O (l) Reaction with sodium carbonate Fizzes Produces carbon dioxide 2 HCOOH (aq) + Na2CO3 (s) 2HCOONa (aq) + H2O (l) + CO (g) Salts of carboxylic acids • • • • Called carboxylates Contain RCOO- group Sodium ethanoate dissolves to give CH3COONa (s) CH3COO- (aq) + Na+ (aq) sodium ethanoate ethanoate ions + sodium ions REMINDER • Parents’ Evening – Thursday 10th February • Coursework due – Monday 14th February Learning objective: 28/04/2017 To introduce esters as products of the reactions of alcohols with carboxylic acids To observe properties of esters To demonstrate the procedure for making an ester on a laboratory scale To explain the purposes of practical techniques involved in the preparation of an ester Complete first section of carboxylic acids and esters sheet Making Esters • • • • Why is it necessary to heat the test tubes? Why should you heat them in a water bath, not directly in a flame? What is the purpose of adding concentrated sulfuric acid to the mixture of the organic acid and the alcohol? Suggest why you poured the mixture into sodium carbonate solution, not just water. Use the example of esters and water to explain what the work ‘immiscible’ means. 1. 2. 3. 4. 5. 6. Add 10 g ethanoic acid and 8 g ethanol to the distillation flask. Swirl and carefully add 2 cm3 concentrated sulfuric acid. Fit a reflux condenser and heat gently for 10 minutes. Cool. Rearrange the apparatus for distillation. Distil off everything up to 82 °C. (Clean the distillation apparatus for step 6.) Transfer the distillate to a tap funnel. Taking the usual precautions to release gases, shake first with sodium carbonate solution to remove acids and then with calcium chloride solution to remove unchanged ethanol. In both instances run off and discard the lower, aqueous layer. Transfer to a small flask. Add a few granules of anhydrous calcium chloride. Stand for a few minutes until the liquid is clear. Redistil from the clean distillation apparatus and collect the fraction boiling in the range 74–79 °C. Structure and naming acid + alcohol ester + water ethanoic acid + methanol methyl ethanoate + water propanoic acid + butanol butyl propanoate + water I C=O I O I H-C-H I Ester Functional group • The oxygens are not directly bonded to the hydrogens • The molecule does not form hydrogen bonds so do not mix well with water • Esters are much more volatile (evaporate more easily) than the alcohol and acid that form them ethanol propanoic acid ethyl propanoate water 1. Heat under reflux preparation Separating aqueous layer purification 2. 3. Fractional distillation purification Drying with anhydrous CaCl2 purification Fractional distillation purification 4. 5. 6. Add 10 g ethanoic acid and 8 g ethanol to the distillation flask. Swirl and carefully add 2 cm3 concentrated sulfuric acid. Fit a reflux condenser and heat gently for 10 minutes. Cool. Rearrange the apparatus for distillation. Distil off everything up to 82 °C. (Clean the distillation apparatus for step 6.) Transfer the distillate to a tap funnel. Taking the usual precautions to release gases, shake first with sodium carbonate solution to remove acids and then with calcium chloride solution to remove unchanged ethanol. In both instances run off and discard the lower, aqueous layer. Transfer to a small flask. Add a few granules of anhydrous calcium chloride. Stand for a few minutes until the liquid is clear. Redistil from the clean distillation apparatus and collect the fraction boiling in the range 74–79 °C. Fats and oils Fats and oils Naturally occuring Animal fat Vegetable oil Marine oil lard suet sunflower oil coconut oil cod liver oil whale oil Homework Find out about the issues around trans fats. • You need to make bullet points on: – The sources, structure and properties of saturated and unsaturated fats – The difference between cis and trans fats in terms of molecular structure – A description and explanation of the effect that cis and trans structures have on properties – Explain how oils are hardened – Explain the problems with the trans fats formed when oils are hardened Fats and oils Fats and oils are built from an alcohol with three -O-H groups. glycerol Systematic name is propane-1,2,3-triol Fats and oils The other components of fat molecules are carboxylic acids such as Stearic acid Systematic name is octadecanoic acid Fats and oils Fats and oils are ESTERS of glycerol and long chain carboxylic acids Fats and oils Removal of water in the condensation reaction gives - The molecular formula shown above suggests that the fat molecule is shaped like an E, but the molecule is actually shaped more like this: Fats and oils Fats are mainly built from carboxylic acids with C-C single bonds. Stearic acid in beef fat Oils have some C=C bonds in the carboxylic acids from which they are made. Carbon – carbon (>C=C<) double bond here Oleic acid in olive oil Fats and oils Oil Double bonds in oil make the molecule less compact. Less tightly packed molecules make oils liquid. Fat Fat molecules pack together more tightly, making fats solid at room temperature. This is like he difference between ldpe and hdpe last year Testing for unsaturation • Carbon-carbon double bonds (>C=C<) decolourise bromine water Fats and oils • that fats and oils are esters of glycerol with fatty acids. • that unsaturated compounds can be recognised by the presence of C= C double bonds.