* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download organic chemistry i
Survey
Document related concepts
Transcript
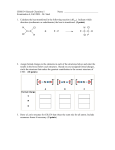
ORGANIC CHEMISTRY I WORK-SHEET 2 BONDING AND HYBRIDIZATION Write the definition of a covalent bond according to the Lewis model. What can you say about each of the carbon-carbon bonds in ethylene (given below) on the basis of Lewis model (only)? C2H4 Write a neutral molecule CH2 assuming that the valence electrons of carbon are configured as follows: 2s2 2p2 Do you expect this molecule to be stable or not stable? Explain your answer. What model did you use to come up with your answer? The molecule CH2 actually exists. Do some research about this molecule and find out about some of its properties as well as its name (the class of compounds it belongs to) Propose an electron configuration for carbon on the basis that carbon makes four bonds, but one of the bonds is stronger than the other three (the latter assumption is not correct) Propose an electron configuration for carbon on the basis that carbon makes four bonds, and all four bonds are equally strong. Carbon orbitals in methane are hybridized orbitals. Why is this terminology used? Bond strength can be predicted using the orbital overlap model. Which makes the stronger bond, a face-to-face overlap or a sideways overlap? Explain your choice of answer Give the terms each type of overlap is referred as. Explain why a sideways overlap is referred to as (your answer in the previous question)? Explain on the basis of bond strength why ethylene is more reactive than ethane. Propose a hybridization model for carbon in acetylene, C2H2. Draw the structure of acetylene and identify each overlap. Explain the relative geometry of each of the p-orbitals for each carbon atom. Changing gears here… How is the bond between C-C different from the bond in H-Cl according to Lewis theory? How would you be able explain the difference using orbital hybridization model in your argument? In the acid-base reaction below 1. insert the lone pair electrons 2. show the species formed (in the right hand side of the equation) 3. identify the acid, base, conjugate acid, and conjugate base 4. use curly arrows to show electron transfers (bond breaking/bond formation) H O H + H Cl The reaction below is between acetic acid and hydrochloric acid. Is this likely to be an acid-base reaction? Explain. H3C 1. 2. 3. 4. O C OH + H Cl insert the lone pair electrons show the species formed (in the right hand side of the equation) identify the acid, base, conjugate acid, and conjugate base use curly arrows to show electron transfers (bond breaking/bond formation)