* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Dementia prescribing: Good practice guidance
Polysubstance dependence wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Atypical antipsychotic wikipedia , lookup
National Institute for Health and Care Excellence wikipedia , lookup
Prescription costs wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Electronic prescribing wikipedia , lookup
Pharmacogenomics wikipedia , lookup
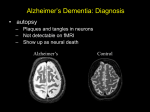
Document name: Dementia prescribing: Good practice guidance. Portfolio Medicines Management Document type: Guidelines Staff group to whom it applies: All clinical staff in OPS Distribution: Intranet How to access: Intranet Issue date: Version 1 - August 2009 Version 1.1 – October 2010 Version 2 July 2012 April 2015 Next review: Approved by: Drug and Therapeutics Sub Committee Developed by: Lynn Haygarth, Chief Pharmacist Director leads: Medical Director Contact for advice: [email protected], www.choiceandmedication.org/swyp Contents 1 What is dementia? .............................................................................................. .. 1 1.1 Alzheimer’s disease ...................................................................................... .. 1 1.2 Dementia with Lewy Bodies.......................................................................... .. 2 1.3 Vascular dementia ........................................................................................ .. 2 1.4 Mini Mental State Examination (MMSE) ....................................................... .. 2 2 Prescribing for symptoms of dementia ................................................................ .. 2 2.1 Prescribing Pathways ................................................................................... .. 5 2.2 How do antidementia drugs work? ............................................................... .. 7 2.3 Side effects ................................................................................................... .. 7 2.4 Drug interactions .......................................................................................... .. 8 2.5 Cardiovascular disease ................................................................................ .. 9 2.6 Respiratory disease ...................................................................................... .. 9 3 Special considerations for prescribing in dementia ............................................. .. 10 3.1 Urinary incontinence ..................................................................................... .. 10 3.2 Sleep disorders............................................................................................. .. 11 3.3 Sexual disinhibition ....................................................................................... .. 11 4 Prescribing for management of behavioural and psychological symptoms of dementia BPSD .................................................................................................. .. 12 4.1 Antipsychotics............................................................................................... .. 13 4.1.1 Use of antipsychotics for people with dementia ................................. .. 13 4.1.2 Choice of antipsychotic ...................................................................... .. 14 4.2 Other Choices............................................................................................... .. 15 4.2.1 Anti-dementia drugs........................................................................... .. 15 4.2.2 Antidepressants ................................................................................. .. 15 4.2.3 Benzodiazepines .............................................................................. .. 15 4.3 Polyprescribing ............................................................................................. .. 16 4.4 Summary points............................................................................................ .. 16 References ................................................................................................................ .. 17 Appendix 1: Antidementia medicines for treatment of dementia of mild to moderate severity ................................................................................................ .. 19 Appendix 2: Appendix 3: Appendix 4: Appendix 5: Appendix 6: Appendix 7: Drugs with antimuscarinic (anticholinergic) effects .............................. .. 22 Use of rivastigmine patch in SWYPFT ................................................ .. 24 Example letter to GP ........................................................................... .. 25 Equality Impact Assessment Tool ....................................................... .. 26 Checklist for review and approval of procedural documents ............... .. 27 Version control sheet ........................................................................... .. 28 ii Abbreviations used in this document AChEIs ACE-R AD BD BP BPSD CNS COPD CYP ECG GP L-dopa LHRH MMSE NICE OM SSRI SWYPFT TCA Z-drugs Acetylcholinesterase inhibitors Addenbrook’s Cognitive Examination Alzheimer’s disease Take twice a day Blood pressure Behavioural and psychological symptoms of dementia Central nervous system Chronic obstructive pulmonary disease Cytochrome Electrocardiogram General Practitioner Levodopa Lutenising hormone releasing hormone Mini mental state examination National Institute for Health and Clinical Excellence Take in the morning Selective serotonin reuptake inhibitor South West Yorkshire Partnership NHS Foundation Trust Tricyclic antidepressant Zaleplon, zopiclone, zolpidem Trust related documents on the intranet Antipsychotics in clinical practice: Guidelines for safe and effective use in adults with schizophrenia (in line with NICE Guidance No 43 2002, updated 2009) version 4, 2012. Guidelines for the pharmacological treatment of anxiety (Incorporating NICE Clinical Guideline 22). Antidepressants in clinical practice: Guidelines for safe and effective use of antidepressants in adults and older people (in line with NICE Clinical Guideline 23 December 2004) Version 5. Hypnotics in Clinical Practice Guidance on the use of hypnotics for the management of insomnia (in line with NICE Guidance No 77 2004). Shared Care Guidelines for anti-dementia drugs donepezil, galantamine, rivastigmine and memantine in Barnsley. Shared Care Guideline for memantine in Calderdale, Kirklees and Wakefield. Patient information leaflets: hypnotics (sleeping tablets), benzodiazepines for anxiety, antipsychotics for the treatment of schizophrenia, antidementia drugs for the treatment of dementia of moderate severity. Patient advice leaflets – donepezil, galantamine, memantine and rivastigmine Medicines Code Section 17 Clinical Queries. iii 1 What is dementia? It is an irreversible syndrome of the brain affecting: Memory Orientation Calculation Judgement Thinking Comprehension Language Initially it starts with mild memory impairment and progresses to behavioural and personality changes to loss of ability to carry out activities of daily living. The prevalence of dementia increases from approximately 0.7% in those aged 60-64 years, doubling every 5 years or so to nearly 40% in those aged 90-95 years. The most common types are: 1 2 3 Alzheimer’s Disease (pure A.D. 38% of dementias) Dementia with Lewy Bodies (contributes about 20% of dementias) Vascular Dementia However 60% of dementias may be mixed. Other diseases can manifest as dementia e.g. Huntington’s Chorea, Parkinson’s Disease dementia, Multiple Sclerosis and AIDS dementia complex. Alcohol abuse may be linked to dementia. 1.1 Alzheimer’s disease This was recognised by Dr Alois Alzheimer in Germany in 1906. He found dense deposits around the nerve cells called amyloid plaques and twisted bands of fibres inside the nerve cells called neurofibrillary tangles. If both are present this gives a diagnosis of Alzheimer’s. These contribute to degeneration of neurons and shrinkage of brain volume. Risk factors include: Increase in age Down’s syndrome Gender - women greater than men Education - if highly educated less likely Family history Head injury More information at www.alzheimers.org.uk 1 1.2 Dementia with Lewy Bodies In this type there is the intracellular inclusion of hyaline mass in the cerebral cortex and substantia nigra. However unlike Alzheimer’s the neurofibrillary tangles are ABSENT. It is particularly associated with fluctuating levels of consciousness. These patients are particularly sensitive to the side effects of antipsychotics and these should be avoided. 1.3 Vascular dementia Onset is classically stepwise following cerebrovascular adverse events due to occlusion of a vessel in the brain following stroke or transient ischaemic attack. Risk factors include hypertension, diabetes and atrial fibrillation. 1.4 Mini Mental State Examination (MMSE) (Caution the written tool is copyright) NICE recommends a mini mental state examination is completed to determine the severity of the dementia. MMSE – score out of 30 21 – 26 Mild 10 – 20 Moderate Less than 10 Severe The symptoms and signs of dementia consist both of features attributable directly to cognitive deficits and also to non-cognitive features some of which include disturbed behaviours (e.g. aggression, wandering and eating disorders) and psychiatric symptoms (e.g. hallucinations, delusions and affective disturbances). These are sometimes referred to as Behavioural and Psychological Symptoms of Dementia (BPSD). For more information refer to Dementia Action Alliance (http://dementiaaction.org.uk/). The ACE-R is also used particularly in Barnsley. 2 Prescribing for symptoms of dementia Formulary choices for symptoms of dementia First line Acetylcholinesterase inhibitors (AChEIs) Donepezil Galantamine If these are not suitable may consider rivastigmine particularly for Parkinson’s patients. Use of rivastigmine patches is restricted. If AChEIs are not tolerated or contraindicated. Memantine 2 Acetylcholinesterase inhibitors (AChEIs) and memantine The AChEIs are the main drugs prescribed for dementia symptoms. Memantine is only available when the AChEIs are contraindicated or not tolerated. These should be used in line with NICE guidance for: People with Alzheimer’s Disease of mild and moderate severity. Non-cognitive symptoms including hallucinations, delusions, anxiety, marked agitation and associated aggressive behaviour. Lewy Body Dementia and mild, moderate or severe Alzheimer’s Disease. People with mixed dementia where Alzheimer’s Disease is considered to be the dominant condition. The following considerations should be taken into account: Baseline monitoring of clinical parameters to eliminate other causes of cognitive impairment should be taken by the GP prior to referral to local memory service. An ECG should be taken prior to commencing treatment. Prescribing must take place in the approved memory service. Consider the circumstances of the service user when choosing an AChEl. If there are no individual considerations or contraindications choose donepezil. Use once a day oral preparation where possible. This helps to facilitate home care visiting once a day. Donepezil is available in an orodispersible (melt in the mouth) tablet. Where liquids are available these require administering twice a day. Take care when prescribing as these are in doses per ml via a dropper. The dose must be titrated slowly starting with the lowest available dose and assess over four weeks for side effects. All service users and carers should have access to the Trust information document on antidementia medicines. (See Appendix 1) Memantine should only be chosen for Alzheimer’s of moderate severity when the AChEIs are not tolerated or contraindicated. When the patient is stabilised on the treatment they may be considered suitable for shared care. o In the case of side effects affecting compliance and functioning, the patients should be referred back to the local memory service. If the MMSE goes below 10 and the diagnosis becomes one of severe dementia the clinician should consider whether to discontinue the treatment or whether continuation will be of benefit. Treatment discontinuation in patients who have responded may cause deterioration; GPs are requested to contact the local memory service for advice. Prescribe generically at all times as there will be cost savings coming through in 2012 with generic products becoming available. 3 Table 1 Information on AChEIs and memantine for symptoms of dementia Drug When to choose Comments Dose Low ≤ £25 Medium £26 - £75 High ≥ £75 £150 Very High ≥ £150 Acetylcholinesterase inhibitors Donepezil Aricept® First line for mild to moderate dementia. Reversible inhibitor of ACHE. Most commonly prescribed. Once a day preparation. Galantamine Reminyl®XL First line and with mixed Alzheimer’s and vascular dementia. Reversible inhibitor of ACHE and a nicotinic receptor agonist. Once a day preparation. Take care when prescribing the liquid to ensure dose is per ml. More side effects reported. Licensed for use in dementia related to Parkinson’s disease. Rivastigmine Exelon® Rivastigmine patches Exelon® Restricted to users not suitable for or tolerant of donepezil and galantamine. Restricted use Swallowing difficulties. Only available via clinical queries mechanism. Glutamate receptor antagonist Memantine For moderate Ebixa ® dementia only either when AChEIs are Contraindicated or not tolerated. Cost per 28 days 5mg and 10mg tablets to swallow and melt in the mouth tablets (orodispersible) Modified release capsules 8mg, 16mg & 24mg, Solution 4mg /mL (liquid given by a dropper) Low Capsules 1.5mg, 3mg, 4.5mg and 6mg. Solution 2mg/ml (liquid given by a dropper) Patches® 4.6mg and 9.5mg. Medium Reduced dose in hepatic 10mg and 20mg impairment. Do not use tablets. in patients with a history of convulsions. Oral solution 10mg/ml as a pump spray Medium Medication errors and inappropriate use of patches has been reported frequently due to lack of patch removal resulting in symptoms of overdose including nausea, vomiting, diarrhoea, hypertension and hallucinations. Low: £120. High High High Medium Can be used in severe dementia in particular for BPSD Further information on the individual products is available at www.medicines.org.uk 4 2.1 Prescribing Pathways Each locality may have a variance of the pathways to suit the needs of the services. In Calderdale, Kirklees and Wakefield prescribing, after initiation, can move to the GP for donepezil, galantamine and rivastigmine. In Barnsley they are subject to a Shared Care Guidelines and can be moved to the GP after four months. Donepezil (Aricept®) Pathway Decide on prescribing and obtain the patient and carer’s agreement, give drug information leaflet and explanation about potential side effects. Prescribing starts at 5mg once daily. Dose increase to 10 mg daily (after four to six weeks) if no side effects. Galantamine (Reminyl®) Pathway Decide on prescribing and get the patient and carers agreement, give drug information leaflet and explanation about potential side effects. Prescribing starts at 8mg MR once daily or 4mg twice daily of liquid 4mg/ml. Doses increases to 16mg daily for month 2 and 3 and to 24mg daily at month 4 if no side effects. Rivastigmine (Exelon®) Pathway Only choose if neither donepezil nor galantamine are suitable for the patient. It comes as both oral and patches which have different pathways. Oral preparations-capsules and liquid Decide on prescribing and get the patient and carer’s agreement, give drug information leaflet and explanation about potential side effects. Consider whether the patient can be prescribed twice a day medicines. Prescribing starts at 1.5mg bd for 2 weeks and increases to 6mg twice daily over 4 months if no side effects. Rivastigmine Patches There has been a serious alert relating to inappropriate use of Exelon patches (Ref Novartis EXE10-047 April 2010). Rivastigmine patches are not generally available and can only be considered via the clinical queries mechanism. For service users with swallowing difficulties it may be suitable to consider the rivastigmine patch. It is important to ensure there is a personal carer who is able to apply the patch daily as per the instructions on the product information. Home care are not able to administer the patch in many areas of the Trust. Complete Appendix 1 – use of rivastigmine (Exelon ®) patch in SWYPFT. A clinical queries form will also require completion. 5 Decide on prescribing and get the patient and carer’s agreement, give drug information leaflet and explanation about potential side effects. Initially 4.6mg applied daily to the upper part of the body for at least 4 weeks. Increase to 9.5mg applied daily to the upper part of the body. Continue on this dose as maintenance dose if tolerated. The prescriber should ensure that the following issues have been considered prior to commencing a patient on the Rivastigmine patch: That adequate monitoring arrangements are in place to ensure: the previously applied patch is removed each day. siting of the patch is rotated between the recommended areas of application. only one patch will be worn at any time. patches are not removed by the patient following application. the skin area is clean, dry and hairless. the patches are changed at the same time each day. The person applying the patch is informed of: how to apply the patch how to remove the patch disposal of the patch action to take if the patch falls off or is removed early action to take if more than one patch is accidentally applied Complete appendix 3. NB if the patient stops treatment for more than 2 days then rivastigmine needs to be introduced at the lowest dose and then increased gradually. Memantine prescribing pathway Only choose when an AChEIs is not tolerated or contraindicated. It is not recommended in patients with severe hepatic impairment. Caution is recommended in patients suffering from epilepsy or with a history of convulsions. Decide on prescribing and get the patient and carer’s agreement, give drug information leaflet and explanation about potential side effects. The dose can be increased by 5mg weekly to 20mg daily if the patient tolerates the memantine. There is treatment initiation pack available to support this use. Week 1 5mg daily Week 2 10mg daily Week 3 15mg daily Week 4 20mg daily Continue on 20mg daily if this dose is tolerated. If the patient has renal impairment with GFR between 5-29 ml/min keep dose to 10mg per day. Avoid if GFR is less than 5ml/min. 6 2.2 How do antidementia drugs work? AChEIs Postulated to provide a beneficial effect by augmenting cholinergic function. Inhibit the enzyme acetylcholinesterase that is responsible for the breakdown of acetylcholine. When the drug inhibits this enzyme the breakdown of acetylcholine is slowed down and therefore cholinergic neurotransmission is increased. Each drug has a very different chemical structure: Donepezil – piperidine derivative. Galantamine – phenanthrene derivative. Rivastigmine – carbamate derivative. Cross-sensitivity would not be expected. Therefore, if a patient develops an allergic reaction such as rash an alternative can be safely tried. Memantine 2.3 Is a NMDA ( N-Methyl-D-Aspartate) receptor antagonist. It targets glutamate which levels are raised in dementia. Excess glutamate leads to overexcitation on the NMDA receptor which releases intra-cellular calcium. High levels of intracellular calcium can lead to cell degeneration and cell death. Side effects AChEIs Diarrhoea, muscle cramps, fatigue, nausea, vomiting, insomnia. Headache, pain, common cold and runny nose abdominal disturbance, dizziness, accident. Urinary incontinence. If these occur reduce the dose and titrate more slowly or change treatment. Rare: syncope, bradycardia, sinoatrial and atrioventricular block. If these occur consider stopping and review the treatment plan. Memantine Common: drug hypersensitivity, somnolence, dizziness, hypertension, dyspnoea, constipation and headache. Uncommon: fungal infections, confusion, hallucinations, gait abnormal, cardiac failure, venous thrombosis/thromboembolism, vomiting and fatigue. 7 2.4 Drug interactions Interacting drugs Anticholinergics (antimuscarinics) e.g. procyclidine, oxybutinin See appendix 3 for full list Cholinomimetics e.g. suxamethonium NSAIDs e.g. ibuprofen, diclofenac Drugs slowing heart rate e.g digoxin, beta blockers CYP2D6 inhibitors e.g. paroxetine, fluoxetine CYP3A4 inhibitors e.g. erythromycin, ketoconazole Inducers of CYP2D6 + CYP3A4 e.g. phenytoin, carbamazepine Concomitant use of N-methyl-Daspartate (NMDA)antagonists such as amantadine, ketamine or dextromethorphan Levodopa, dopaminergic, agonists Donepezil Galantamine Rivastigmine Potential antagonistic effect, monitor for reduced efficacy of either drug. Also may worsen the course of the illness Memantine May worsen course of illness Effects may be enhanced Potential additive effect No effect likely Increased risk of gastric irritation and GI bleed No effect likely Potential additive effect, monitor for side effects (e.g. bradycardia) No effect likely Donepezil levels possibly increased* Donepezil levels possibly increased* Donepezil levels possibly reduced** No effect likely Galantamine levels possibly increased* Galantamine levels possibly increased* Galantamine levels possibly reduced ** No effect likely No effect likely No effect likely No effect likely No effect likely No effect likely Increased risk of convulsions No effect likely should be avoided as there is a risk of CNS toxicity Effects may be enhanced *Dose reduction not necessary unless side effects occur. ** Interaction may not be clinically significant, but should be considered if lack of efficacy occurs. NB It is unlikely that any of the antidementia drugs at therapeutic doses will affect the metabolism of other medications. Always check SPC when prescribing 8 2.5 Cardiovascular disease In patients with pre-existing cardiovascular disease such as sick sinus syndrome and other supraventricular cardiac conduction conditions the manufacturers advise caution. May cause bradycardia due to their vagotonic effects, and arrhythmias. May also have variable effects on BP eg raised or lowered BP. Underlying hypotensive conditions may be exaggerated – leading to falls. Memantine may be considered as a suitable choice however there is only limited data in patients with recent MI, congestive heart failure or uncontrolled hypertension. Patients should be closely monitored. 2.6 Respiratory disease In patients with respiratory disease the manufacturers recommend that AChEIs should be used with caution in patients with a history of asthma or COPD. This appears to be based on the theoretical risk that cholinomimetics can cause bronchoconstriction and bronchospasm, rather than actual reports of problems in this patient group. If used then there must be increased monitoring of respiratory function, especially during titration. Lower starting doses and slower titration could be used in patients with severe or poorly controlled respiratory disease. 9 3 Special considerations for prescribing in dementia Polyprescribing should be minimized wherever possible and patients prescribed more than four drugs should undergo a medication review. This can be supported by the local pharmacy team. Drugs with anticholinergic effects can both increase cognitive decline and antagonize effects of the treatments. These include drugs for urinary incontinence, antimuscarinic/anticholinergics and tricyclic antidepressants. (Appendix 2 for further information) 3.1 Urinary incontinence Drugs for bladder problems are often used in older patients. In addition, urinary incontinence can be a side effect of acetylcholinesterase treatment. Those commonly prescribed are: • Oxybutynin, tolterodine, and trospium – – – – Licensed to treat urinary frequency, urgency and incontinence. None are specific to the bladder. All may cause constipation, tachycardia, and CNS effects. There have still been case reports of cognitive decline in patients with pre-existing dementia which reversed on withdrawal of the drug. It is not an absolute contraindication to use if there is a definite clinical need. Patients should be monitored very closely. Interaction should be considered if the patient’s cognitive function declines or bladder symptoms worsen. There are case reports of the combination being used successfully in patients distressed by the symptoms of incontinence. 3.2 Sleep Disorders In patients with dementia the sleep-wake cycle is often disturbed. It is important to avoid benzodiazepines (even short-acting) as they can cause “hang-over” the next morning. They also cause confusion and ataxia (increased risk of falls). Longer acting benzodiazepines e.g nitrazepam are a particular risk. A short course of a Z-drug may be helpful e.g. 3.75mg zopiclone. This should be regularly reviewed and only continued if essential. Other sleep hygiene measures should be considered. (See SWYPFT hypnotic policy) Unfortunately many patients may already be prescribed benzodiazepines or z drugs. It is important not to stop abruptly. If these are given there is an increased risk of slips, trips and falls. It is most important to ensure they are only administered when the patient is actually ready for bed. 10 Dementia may impair driving performance and the ability to use machinery. Use of hypnotics may cause further impairment. 3.3 Sexual Disinhibition This has a prevalence rate of 2-17% in this group of patients. It can manifest as inappropriate nudity, public masturbation or stripping, obscene sex language, or propositioning others. There is a significant association with severity of dementia. There is no licensed drug for treating dementia-related sexual disinhibition. Preparations that may be considered under the clinical queries mechanism to be used off licence include: Anti-androgens (e.g. cyproterone), oestrogens, LHRH analogues, serotonergics and gabapentin. 11 4 Prescribing for management of behavioural and psychological symptoms of dementia BPSD Many different factors may be associated with behavioural problems in patients with dementia: Physical illness including: poorly controlled pain diabetes with impaired glucose metabolism dehydration hypoxia electrolyte disturbances heart failure delirium Prescribed medicines These may cause psychotic symptoms in elderly during use or on withdrawal: Benzodiazepines Anti-Parkinson drugs - L Dopa, procyclidine Anti-arrhythmics – digoxin, propranolol Anti-inflammatories – aspirin, indomethacin Anticonvulsants – carbamazepine, phenytoin Steroids - prednisolone Environmental factors noisy and over stimulating environments social isolation visual and auditory sensory impairments Age related neurotransmitter changes (acetylcholine, dopamine, noradrenaline and serotonin), damage to specific brain regions responsible for emotional activity (parahippocampal gyrus, dorsal raphe and locus coeruleus) and cortical hypometabolism have also been proposed as possible neurobiological causes (Lanari et al 2006). It is also important to consider: Does the patient have communication difficulties eg dysphasia. Could pain control be improved? Is it delirium or dementia? - These often exist together so treat the delirium and often the behavioural symptoms will subside. Is it depression which is common in dementia. For more information about assessing symptoms please refer to: Best practice guide: optimising treatment and care for people with BPSD toolkit (Alzheimer’s society) http://nww.swyt.nhs.uk/drug-therapeutics tag/Documents/qipp/A_best_practice_guide_for_health_and_social_care_profes sionals.pdf Reducing the use of antipsychotic drugs toolkit http://nww.swyt.nhs.uk/drug-therapeuticstag/Documents/qipp/Reducing_the_use_of_antipsychotic_drugs.pdf 12 4.1 Antipsychotics There has been increasing concern over the past few years about the use of antipsychotics in dementia. Prior to 2000 Typical antipsychotics were common such as haloperidol, thioridazine and promazine but older people were more sensitive to their side-effects such as movement disorders and QT prolongation. Atypicals became more commonly prescribed. In 2004 The CSM reported an apparent 2 to 3 fold increase in risk of cerebrovascular event in people with dementia prescribed olanzapine and risperidone. In 2005 It became apparent that no antipsychotic was safer than any other when it was reported a 1.7 fold increase in mortality with the typicals due to heart failure, sudden death and pneumonia. NICE/SCIE guideline 42 Nov 2006 recommends the use of pharmacological intervention in the first instance only if the patient is severely distressed or there is an immediate risk of harm to the person or others. The National Dementia Strategy advocates only the appropriate use of antipsychotic medication for people with dementia. Banerjee in his ‘Time for Action’ report states that across the country 180,000 people are being treated with antipsychotics of which 36,000 will derive benefit but an additional 1,600 cerebrovascular effects (of which half are severe) and 1,800 deaths occur than would be expected. Antipsychotics are being use too often first line in response to behavioural difficulty such as agitation, aggression and wandering rather than second line after other non-pharmacological approaches have failed. 4.1.1 Use of antipsychotics for people with dementia a. b. c. d. e. g. Antipsychotics should not be first line except in circumstances of extreme risk or harm. Psychological approaches should be used in the first instance. Identify treatable causes of BPSD (eg. delirium, pain or depression) and prescribe accordingly. Where antipsychotic medication is considered the client (if capacity to consent), relative or carer should be involved in the decision after having had the information about the potential positive and negative effects. A patient information leaflet should be provided. The final prescription will be a ‘best interests’ decision having identified a clear target symptom An atypical is preferred over a typical using the lowest effective dose for shortest period of time, ideally less than 12 weeks. Once initiated continuation should be reviewed monthly and reduction or cessation actively considered. A record on RiO should include the discussion about the risks and benefits which took place with the client/relative/carer, the indication for the prescription, alternatives considered and plans for review, reduction and cessation. 13 h Where the use in unlicensed (see table below) an unlicensed use form (section 17 medicines code) should be completed. The responsibility for prescribing will remain with the prescriber. Refer to points to consider before prescribing: Dementia Prescribing Guidance: http://nww.swyt.nhs.uk/drug-therapeuticstag/Documents/qipp/Checklist%20Guidance%20Booklet%20Print.doc Antipsychotic in Dementia Checklist : http://nww.swyt.nhs.uk/drug-therapeuticstag/Documents/qipp/Antipsychotic%20in%20Dementia%20checklist.doc 4.1.2 Choice of antipsychotic Antipsychotic Starting Dose Optimal Comment dose ONLY USE WHEN NO OTHER STRATEGY IS AVAILABLE Risperidone 250micrograms 1mgLicensed for use 6 weeks only to 2mg for treatment of persistent 500micrograms aggression in patients with moderate to severe Alzheimer’s disease unresponsive to nonpharmacological approaches and where there is a risk of harm to self or others. Evidence of increased risk of stroke. May cause stiffness. Caution in combination with furosemide. Amisulpride 12.5mg -25mg 50mg Unlicensed Risk of stiffness, 100mg limited evidence of response. Haloperidol 500micrograms 2mg- 3 Licensed only for agitation and mg restlessness. Risk of stiffness. The following drugs, which are not licensed for use in dementia, have been prescribed in the past but are no longer recommended. Service users taking these drugs need to be reviewed. Olanzapine 2.5mg 5mg – Unlicensed. Evidence of 7.5mg increased risk of stroke. Not recommended by Trust or APC Quetiapine 12.5mg to 25mg 100mg - Unlicensed, not recommended no suitable 300mg by Trust or APC – on grey list. formulation or Limited evidence of response. preparation to give 12.5mg exists In special circumstance e.g. patients with parkinsonian symptoms, or severe EPSEs. Ensure a note is made on RiO and an unlicensed use form completed. 14 Many patients are already prescribed antipsychotics which may be continued inappropriately. The Trust have developed a series of tools to support the review of antispychotics in dementia and these are available on the trust website www.swyt.nhs.uk/ Do not hesitate to contact the Trustwide medicines information department for support when reviewing patients prescribed antipsychotic medication for dementia. Tel 01924 327619 email [email protected] 4.2 Other Choices 4.2.1 Anti-dementia drugs There is some evidence to recommend the AChEIs. Given their mechanism of action, these drugs would seem to be a logical choice when considering pharmacological options for the management of behavioural disturbance in dementia. More specifically there have been a number of studies of individual drugs showing benefit in the management of behavioural problems in dementia including donepezil, galantamine, rivastigmine (eg Feldman et al., 2001; Holmes et al., 2004; Monsch et al., 2002; Gabelli, 2003; Finkel, 2004; Cummings et al 2006). Use in these circumstances is outwith the NICE guidance although the use would be licensed. If using these drugs in particular follow the guidance in section 2. In addition it is important to ensure the target symptoms are identified and there is noted review and discontinuation if there is no response to treatment. NB: Memantine (Ebixa®) should only be prescribed for BPSD in line with the shared care guidelines 4.2.2 Antidepressants Although depression can be difficult to diagnose in dementia there is some evidence of benefit particularly with SSRIs and trazodone. Consider using antidepressants for treatment of co-morbid depressive symptoms in dementia (e.g. citalopram or mirtazapine). As citalopram is an SSRI, monitor for emergence of gastric symptoms. Consider trazodone for patients with depressive symptoms and dementia associated with agitation. Tricyclic antidepressant use is not recommended. See SWYPFT antidepressants in clinical practice. 4.2.3 Benzodiazepines As a group these drugs can cause significant sedation, postural hypotension and memory impairment so they need to be used with care. If choosing a benzodiazepine use one with a short half life to prevent accumulation. Lorazepam is widely used but it should only be prescribed short term because of the risks of side-effects, especially falls and of tolerance developing. It should only be used in low doses e.g. 500 micrograms to 1mg daily. The 15 prescription should be regularly reviewed and reduced and stopped wherever possible. Diazepam has also been used but this should be avoided as it has a long half life and accumulation can occur in older people. 4.3 Polyprescribing Many patients are already prescribed medicines for physical illness and adding in psychotropic medicines must be done with particular care. Consider the presentation –could it be a side effect? swallowing difficulties with antipsychotics stiffness with antipsychotics sedation with trazodone and lorazepam gastric irritation with an SSRI Review existing treatment before prescribing other medicines. Reviewing medication choices: Review ideally after 4-6 weeks of commencement of acetylcholinesterase inhibitor to check for efficacy and side effects. Routine assessments should be repeated 3 to six monthly. (BNF, NICE) Antipsychotic use is only advised for short term use (up to 6 weeks). 4.4 Summary points At all times consider the circumstances of the patient. Antipsychotics are not first line treatment. Only prescribe antipsychotics for target symptoms, at low doses for short term treatment. Do not prescribe procyclidine. Do not discharge to GP on antipsychotics without a follow up care plan. Avoid tricyclic antidepressants. Avoid anticholinergics. If essential use a short course of z-drugs for sleep problems. Only use benzodiazepines with a short half life for short term treatment. Never use more than one benzodiazepine. Never discharge patients on long course of z drugs and benzodiazepines. 16 References Banerjee S (2009) The use of antipsychotic medication for people with dementia: Time for action. British National Formulary (BNF) Number 62 September 2011 CSM (2004) Committee on Safety of Medicines. Atypical antipsychotic drugs and stroke. Curran, S. and Wattis, J.P.W. (2004) Practical Management of Dementia – A MultiProfessional Approach, Radcliffe Medical, Oxford. Drug Safety Update Volume 2, Issue 8 March 2009 from MHRA and CHM. Food and Drug Administration – US FDA (2005) Off-label use of atypical antipsychotics linked to increased mortality in the elderly. www.medscape.com Knapp et al Dementia UK 2007: The full report dementia UK report. London Alzheimer’s Society. Lanari-Alessia, Amenta-Fncesco et al.(2006) Neurotransmitter deficits in behavioural and psychological symptoms of Alzheimer’s disease. Mechanisms of ageing and development, 127(2), 158-65. MeRec Stop Press blog no 847 NICE/SCIE 2006 : Guideline to improve care of people with dementia. NICE Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease (March 2011). www.nice.org.uk National Prescribing Centre: Key Therapeutic Topics 2010/11 Medicines management options for local implementation. Novartis Direct healthcare professional communication EXE10-047 March 2010 Rosler M (2002) The efficacy of cholinesterase inhibitors in treating the behavioural symptoms of dementia. International Journal of Clinical Psychiatry127 (Suppl.), 20-36. Series H & Degano P. Hypersexuality in dementia. Advances in psychiatric treatment 2005;11:424-431 Working Group for the Faculty of the Psychiatry of Old Age of the Royal College of Psychiatrists, (2004) Summary guidance for the management of behavioural and psychiatric symptoms in dementia and the treatment of psychosis in people with history of stroke/TIA. www.rcpsych.ac.uk/college/faculty/oap 17 Antipsychotics in Dementia checklist: http://nww.swyt.nhs.uk/drug-therapeuticstag/Documents/qipp/Antipsychotic%20in%20Dementia%20checklist.doc Reducing Antipsychotic flowchart: http://nww.swyt.nhs.uk/drug-therapeuticstag/Documents/qipp/Reducing%20Antipsychotic%20use%20in%20Psychiatric%2 0Illness%20Flowchart.doc Flowchart for quetiapine liquid: http://nww.swyt.nhs.uk/drug-therapeuticstag/Documents/qipp/Switching%20from%20Quetiapine%20Liquid.doc 18 Appendix 1 Antidementia medicines for the treatment of dementia of mild to moderate severity What are antidementia medicines? These medicines are used to help improve memory and other brain functions and prevent the deterioration caused by Alzheimer’s disease (also known as dementia) and other similar conditions. The ones most commonly prescribed are also known as acetylcholinesterase inhibitors (AChEIs). Another drug, memantine, may be prescribed if the AChEIs are not suitable for the person. They are used to help people cope with activities of daily living and help slow down further memory loss. They may also help with other problems which may be associated with dementia such as hallucinations (seeing or hearing things), delusions (believing things that aren’t true), anxiety, agitation and aggressive behaviour. They come in different forms including tablets, capsules, liquids and patches. They are known by two names –one is the name given by the manufacturer and the other is the “generic” name that the medicine is registered by. Acetylcholinesterase inhibitors (AChEIs) Generic name Manufacturers name Donepezil Forms Aricept® 5mg and 10mg tablets to swallow and melt in the mouth tablets (orodispersible) Galantamine Reminyl®XL Modified release capsules 8mg, 16mg & 24mg, Solution 4mg /mL (liquid given by a dropper) Rivastigmine Exelon® Capsules 1.5mg, 3mg, 4.5mg and 6mg and oral solution 2mg/ml (liquid given by a dropper) Patches® 4.6mg and 9.5mg, Memantine is given when the AChEIs are not suitable for the patient Memantine Ebixa ® 5mg,10mg and 20mg tablets Oral solution 10mg/ml (available as a pump spray) 19 How are antidementia drugs prescribed? These are normally prescribed by a specialist memory service. There is one in Barnsley, Dewsbury, Halifax, Huddersfield and Wakefield. If the GP thinks you will benefit from seeing the local memory service you will be referred. If the doctor or nurse at the memory clinic thinks you may benefit you will be prescribed an antidementia medicine. Also geriatricians, neurologists do start some patients on antidementia drugs. Before starting treatment you will have a memory test. This is known a mini-mental state examination (MMSE) or an ACE-R. This will be repeated in the future to see how you are doing and whether you are getting any benefit from the medicine. If you are not getting any benefit the memory service may discuss stopping the medicine. These medicines are always prescribed at a low starting dose to try and stop side effects occurring. The dose is normally increased after a month if you have no troublesome side effects. Are antidementia drugs safe to take? You will need to have blood tests and an ECG, or heart trace, before starting to take the medicines. This is to check that it is safe for you to take the medicine. If you have any heart problems these medicines may not be suitable for you. If you have asthma or respiratory problems you will monitored closely as there is a small risk that this could be made worse. You will also be asked what other medicines you are taking to check there will be no risks of any interactions. You should also say if you are taking any other medicines and health supplements that you have bought so these can be checked. Benefits of antidementia drugs They help slow down the rate of memory loss and allow you to continue to carry out activities of normal daily living. They may also help with some of the distressing symptoms of anxiety, irritability, hallucinations (seeing or hearing things) and delusions (believing things that are not true). How will I get my medicines? At the beginning and for at least 2 months you will be given a prescription from the doctor or nurse in the memory clinic to take to your local chemist. It is a good idea to use the same chemist as they will know what other medicines you are taking. If you are doing well then a letter will be sent to your GP asking him to prescribe for you. If agreed, you can collect the prescription from there. 20 Side effects of antidementia drugs These are usually mild and many wear off after a short time. Side effects include abdominal disturbance nausea, vomiting, diarrhoea – loose bowel movements muscle cramps, fatigue or tiredness, insomnia – not sleeping properly at night headache, pain common cold or runny nose dizziness, accident urinary incontinence (needing to go to the toilet a lot to empty the bladder). If these occur the dose may be reduced or the treatment changed. Very rarely side effects can affect the heart – if they do the treatment will be reviewed. It is important to report any side-effects to your doctor or nurse. Further information – For further more detailed information please discuss with a doctor, non-medical prescriber or pharmacist Information leaflets on individual antidementia medicines can be found on the South West Yorkshire Partnership NHS Foundation Trust intranet which a member of staff can access for you. More information can be found at www.alzheimers.org.uk and www.choiceandmedication.org/swyp/ You may also find the information and links on NHS Direct website useful. www.nhsdirect.nhs.uk Do not stop or make changes to any medicines without talking to a healthcare professional. 21 APPENDIX 2 A large population-based study, in participants with normal or mildly impaired cognition, has shown that the use of medications with anticholinergic activity, increased the risk of cognitive decline (as measured by the MMSE) and increased the risk of mortality over 2 years, especially in the older adult population. Further research is needed to confirm and extend these findings, in particular the effect on mortality of anticholinergic burden and of different doses of medicines with anticholinergic activity but the table below provides a useful guide to the anticholinergic burden (ACB) for a wide range of medication. Drugs on the Anticholinergic Burden (ACB) scale (A total ACB scale score of three or more is considered clinically relevant) ACB Score 1 (mild) (severe) Alimemazine Alprazolam Alverine Atenolol Beclometasone dipropionate Bupropion hydrochloride Captopril Chlorthalidone Cimetidine hydrochloride Clorazepate Codeine Colchicine Dextropropoxyphene Diazepam Digoxin Dipyridamole Disopyramide phosphate Fentanyl Fluvoxamine Furosemide Haloperidol Hydralazine Hydrocortisone Isosorbide preparations Loperamide Metoprolol Morphine Nifedipine Prednisone/Prednisolone Quinidine Ranitidine Theophylline Timolol maleate Trazodone Triamterene Warfarin ACB Score 2 (moderate) ACB Score 3 Amantadine Belladonna alkaloids Carbamazepine Cyclobenzaprine Cyproheptadine Loxapine Meperidine Methotrimeprazine Molindone Oxcarbazepine Pethidine hydrochloride Pimozide Amitriptyline Amoxapine Atropine Benztropine Chlorpheniramine Chlorpromazine Clemastine Clomipramine Clozapine Darifenacin Desipramine Dicyclomine Diphenhydramine Doxepin Flavoxate Hydroxyzine Hyoscyamine Imipramine Meclizine Nortriptyline Orphenadrine Oxybutynin Paroxetine Perphenazine Procyclidine Promazine Promethazine Propentheline Pyrilamine Scopolamine Thioridazine (withdrawn) Tolterodine Trifluoperazine Trihexyphenidyl Trimipramine 22 Notes: 1. Certain medicines eg Risperidone (mild ACB), Quetiapine (severe ACB) and Olanzapine (severe ACB) were licensed after 1990 and therefore not prescribed to the original CFAS cohort. 2. Brand names may conceal generic drug names. 3. Some combination medicines contain anticholinergic drugs. 4. This list is indicative and some related medicines were taken by patients in the CFAS study; if appropriate these related medicines were given an ACB score based on the ACB of the related medicine in the Aging Health publication Anticholinergic Medication Use and Cognitive Impairment in the Older Population: The Medical Research Council Cognitive Function and Ageing Study Chris Fox,_ MD,a Kathryn Richardson,_ MSc,b Ian D. Maidment, MA,cd George M. Savva, PhD,e Fiona E. Matthews, PhD,f David Smithard, MD,gh Simon Coulton, MSc,d Cornelius Katona, MD 23 Appendix 3 Use of rivastigmine patch in South West Yorkshire Partnership Foundation Trust The patch should be chosen for use when there is a carer who is able to apply the patch to the patient every day. The patch should be applied daily at approximately the same time of day. Prior to prescribing the patch please ensure that there is an appropriate carer who is able to change the patch daily in line with the instructions from Novartis – Exelon patches. Please complete the following when prescribing rivastigmine patches. Patient name: ___________________________________________________________________ Consultant : ___________________________________________________________________ Rio/Hospital Number: ____________________________________________________________ I have identified a carer to apply the patches. Information on application of the patches has been issued and understood. Information given Rivastigmine (Exelon) patch Q & A guidance. Exelon patch including application guidance and chart. I confirm this information has been received and understood. Name of memory service clinician: ____________________________________________ Signature of clinician:_______________________________________________________ Date: ____________________________________________________________________ Please fax a copy to the Chief Pharmacist’s office (01422 281568) and retain a copy in the notes or detail on electronic record. An electronic copy can be sent to [email protected] (pharmacy secretary) 24 Appendix 4 Example letter to GPs GP letter Our Ref: NHS No: Date PRIVATE AND CONFIDENTIAL Dear Dr Re: Diagnosis: (please specify type of dementia where known and secondary diagnosis eg depression) Care Coordinator: Following discussion with Dr…name and job title……….. the following medication has been prescribed/recommended for your patient (delete as appropriate) Medication recommended: Reason for prescribing/target symptom: (identify nature of symptom eg agitation, depression hallucinations etc) Further Monitoring Arrangements: A prescription for Based on the current evidence in relation to prescribing antipsychotic drugs in dementia we recommend an ongoing prescription up to 3 months only. Medication should only be continued beyond this time if the target symptom for which medication was prescribed recurs. months/days has been provided. Yours sincerely c.c. Dr………………………… 25 Appendix 5- Equality Impact Assessment Tool To be completed and attached to any policy document when submitted to the Executive Management Team for consideration and approval. Yes/No 1. Comments Does the policy/guidance affect one group less or more favourably than another on the basis of: Race Ethnic origins travellers) NO (including gypsies and NO Nationality NO Gender NO Culture NO Religion or belief NO Sexual orientation including lesbian, gay and bisexual people NO Age NO Disability - learning disabilities, physical disability, sensory impairment and mental health problems Recommendations are given for treatment in older people and also for different illness types eg lewy body dementia which may differ from the general recommendations. Currently have no patient information leaflets in any other media other than type face. 2. Is there any evidence that some groups are affected differently? YES 3. If you have identified potential discrimination, are any exceptions valid, legal and/or justifiable? YES 4. Is the impact of the policy/guidance likely to be negative? NO 5. If so can the impact be avoided? N/A 6. What alternatives are there to achieving the policy/guidance without the impact? N/A Different patients may respond differently to anti dementia drugs. ` 7. Can we reduce the impact by taking different N/A action? If you have identified a potential discriminatory impact of this policy, please refer it to the Director of Corporate Development or Head of Involvement and Inclusion together with any suggestions as to the action required to avoid/reduce this impact. For advice in respect of answering the above questions, please contact the Director of Corporate Development or Head of Involvement and Inclusion. 26 Appendix 6- Checklist for the Review and Approval of Procedural Document To be completed and attached to any policy document when submitted to EMT for consideration and approval. Title of document being reviewed: 1. 2. Comments Title Is the title clear and unambiguous? YES Is it clear whether the document is a guideline, policy, protocol or standard? YES Good practice guidance Rationale Are reasons for development of the document stated? 3. Yes/No/ Unsure YES Development Process Is the method described in brief? YES Are people involved in the development identified? YES Do you feel a reasonable attempt has been made to ensure relevant expertise has been used? YES Is there evidence of consultation with stakeholders and users? YES D&T membership and Area Prescribing Committee Old age psychiatrists 4. 5. 6. 7. Content Is the objective of the document clear? YES Is the target population clear and unambiguous? YES Are the intended outcomes described? YES Are the statements clear and unambiguous? YES Evidence Base Is the type of evidence to support the document identified explicitly? YES Are key references cited? YES Are the references cited in full? YES Are supporting documents referenced? YES Approval Does the document identify which committee/group will approve it? YES If appropriate have the joint Human Resources/staff side committee (or equivalent) approved the document? N/A Dissemination and Implementation Via trust Intranet 27 Title of document being reviewed: 8. Is there an outline/plan to identify how this will be done? YES Does the plan include the necessary training/support to ensure compliance? N/A Have archiving arrangements for superseded documents been addressed? YES N/ Process to Monitor Compliance and Effectiveness Are there measurable standards or KPIs to support the monitoring of compliance with and effectiveness of the document? Is there a plan to review or audit compliance with the document? 10. 11. Comments Document Control Does the document identify where it will be held? 9. Yes/No/ Unsure Use of clinical queries mechanism YES Prescribing Observatory for Mental Health Audit Plan Review Date Is the review date identified? YES Is the frequency of review identified? If so is it acceptable? YES Overall Responsibility for the Document Is it clear who will be responsible implementation and review of the document? Appendix 7- Version YES Control Sheet This sheet should provide a history of previous versions of the policy and changes made Version Date Author Status Comment / changes 1 August 2009 Lynn Haygarth Written in line with NICE guidance 1.1 October 2010 Lynn Haygarth Updated in line with new guidance for rivastigmine patches 2 June 2012 Lynn Haygarth Changes due to revised NICE guidance and updated to include Barnsley and use of memantine. Drugs on the Anticholinergic Burden (ACB) scale. 28