* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chemical Foundations
Survey
Document related concepts
Adenosine triphosphate wikipedia , lookup
Metalloprotein wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Photosynthesis wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Transcript
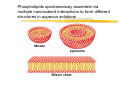
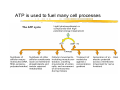
Chapter 2 Chemical Foundations The Chemicals of Life The Chemicals of Life (b) Macromolecules (23%) Covalent bonds and noncovalent interactions The “glue” that holds macromolecules together: a Covalent bonds a Noncovalent interactions: ionic bonds hydrogen bonds van der Waals interactions hydrophobicity-driven interactions Covalent bonds a Formed when two different atoms share electrons in the outer atomic orbitals a Each atom can make a characteristic number of bonds (e.g., carbon is able to form 4 covalent bonds) a Covalent bonds in biological systems are typically single (one shared electron pair) or double (two shared electron pairs) bonds Asymmetric carbon atoms are present in most biological molecules a Carbon atoms that are bound to four different atoms or groups are said to be asymmetric a The bonds formed by an asymmetric carbon can be arranged in two different mirror images (stereoisomers) of each other a Stereoisomers are either right-handed or left-handed and typically have completely different biological activities a Asymmetric carbons are key features of amino acids and carbohydrates Water is a polar molecule a a a a Electrons are not distributed equally Electrons have higher affinity for the oxygen Polar molecules have a dipole moment Essential for hydration of ions α and β glycosidic bonds link monosaccharides Noncovalent bonds a Several types: hydrogen bonds, ionic bonds, van der Waals interactions, hydrophobic bonds a Noncovalent bonds require less energy to break than covalent bonds a The energy required to break noncovalent bonds is only slightly greater than the average kinetic energy of molecules at room temperature a Noncovalent bonds are required for maintaining the threedimensional structure of many macromolecules and for stabilizing specific associations between macromolecules Multiple noncovalent bonds can confer binding specificity The hydrogen bond underlies water’s chemical and biological properties Molecules with polar bonds that form hydrogen bonds with water can dissolve in water and are termed hydrophilic Ionic bonds a Ionic bonds result from the attraction of a positively charged ion (cation) to a negatively charged ion (anion) a The atoms that form the bond have very different electronegativity values and the electron is completely transferred to the more electronegative atom a Ions in aqueous solutions are surrounded by water molecules, which interact via the end of the water dipole carrying the opposite charge of the ion Van der Waals interactions are caused by transient dipoles When any two atoms approach each other closely, a weak nonspecific attractive force (the van der Waals force) is created due to momentary random fluctuations that produce a transient electric dipole Hydrophobic bonds cause nonpolar molecules to adhere to one another Nonpolar molecules (e.g., hydrocarbons) are insoluble in water and are termed hydrophobic Since these molecules cannot form hydrogen bonds with water, it is energetically favorable for such molecules to interact with other hydrophobic molecules This force that causes hydrophobic molecules to interact is termed a hydrophobic bond Phospholipids are amphipathic molecules Phospholipids spontaneously assemble via multiple noncovalent interactions to form different structures in aqueous solutions Chemical balance determines which bonds can be formed Copyright (c) by W. H. Freeman and Company Energy in biological systems a Most important forms of energy in a cell: `Chemical potential energy `Energy of concentration gradient `Electric potential energy a All forms of energy are interconvertable a Energy unit in biochemistry: kcal = Cal = 1000 cal a Direction of chemical reaction: towards minimal free energy ΔG [cal/mole] = Gproducts – Greactants Rate of a chemical reaction depends on the activation energy Enzymes accelerate biochemical reactions by reducing transition-state free energy Cellular processes are driven by ATP hydrolysis a All forms of energy are interconvertable a Many chemical reactions are energetically unfavorable (ΔG > 0) and will not proceed spontaneously a Cells can carry out such a reaction by coupling it to a reaction that has a negative ΔG of larger magnitude a Energetically unfavorable reactions in cells are often coupled to the hydrolysis of adenosine triphosphate (ATP), which has a ΔGº′ = -7.3 kcal/mol a The useful free energy in an ATP molecule is contained in phosphoanhydride bonds ATP is used to fuel many cell processes The ATP cycle At the completion of this lecture you should be able to: a Describe the different kinds of bonds that hold biological macromolecules together a Understand how the variability and complexity of organic carbon-based molecules provided (evolution) the basis for the development of life a Describe and draw the structure of micelles, liposomes, and bilayer sheets a Declare how enzymes accelerate biochemical processes