* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cyr, Kelly - American Academy of Optometry
Survey
Document related concepts
Transcript
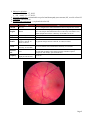
Kelly Cyr American Academy of Optometry Residents Day Case Report Application September 1, 2009 Title: One large blind spot among a sea of dots Abstract: 43 year-old white hyperopic female presents with left eye pain, photophobia, photopsia, metamorphopsia, and visual field loss. Exam findings reveal a left temporal hemianopsia, mild vitiritis, multiple chorioretinal lesions, and exuberant disc edema. I. Case History Patient demographics 43 year old white female of Italian and Portugese descent presents to the eye clinic on consult from neurology. She is currently undergoing a demyelinating disease workup due to reported optic neuritis in the left eye and is experiencing visual symptoms. Chief complaint Ocular complaints - Patient reports the following symptoms which began about 6-7 weeks prior to presentation in our clinic: Pain OS: 10/10, sharp, stabbing, lasted for about 45 minutes at first onset; now occurs for seconds at a time and only about 1-2x/day Photosensitivity: patient has longstanding history, but reports is worse when pain began OS>OD Photopsias/metamorphopsias: patient reports experiencing a “tunnel of lights” at the onset of symptoms, lasted a few days then went away. She no longer notices these lights; however, she now feels as though her vision is like “looking through the reflections of a pool” (rippling of water). She reports that she is unsure which eye this is occurring in and it occurs even when her eyes are closed. Field loss: patient reports she has difficulty viewing left of fixation. She thinks this is more in the left eye, but reports slight decrease in the left visual field in the right eye as well. Patient is unsure whether vision has decreased in either eye. Systemic associations: At onset, patient began experiencing tingling in the back of her head as well as sharp pains in her fingers and toes. The patient has fibromyalgia, but reports these pains are different. She also reports leg numbness and increased fatigue. She feels short of breath and that her heart “flutters”. Headaches: pain is 2-3/10, more frontal and behind the eyes, comes and goes Rash: rash on right arm which she was told was eczema – reports resolution with steroid cream Ocular, medical history Ocular History: Denies the following: history of patching, redness to either eye, ocular trauma or ocular surgery Last eye exam: 1 week prior to presentation: Disc edema OS with left hemifield defect Multiple RPE window defects OS Non-specific white matter changes on MRI 2 months prior to presentation: High hyperopia, astigmatism, presbyopia OU Photophobia OU, longstanding Ocular health WNL OU Note: Given the lack of chorioretinal findings on examination two months prior to presentation with optic disc edema, it appears that the disc edema and chorioretinal lesions are temporally related. Page 1 Medical History: Review of patient’s medical history was significant for the following: Hypertension Hyperlipidemia Iron deficiency anemia Obesity Allergic rhinitis Hearing loss/subjective tinnitus Gastroesophageal reflux disease (GERD) Sluggish bowel syndrome Abdominal pain Low back pain Myositis Carpal tunnel syndrome Fibromyalgia Multiple pulmonary emboli, currently on anti-coagulation treatment (warfarin) Anti-phospholipid antibody Menorrhagia Hysterectomy, 4 months prior Sexual trauma Anxiety/major depressive disorder/post-traumatic stress disorder Medications Multivitamins Bisoprolol (5mg po QHS) Simvastatin Ferrous sulfate (325 mg po daily) Lorazepam (2mg po BID) Sertraline Quetiapine fumarate Omeprazole (20 mg po daily) Enoxaparin injection (140mg q12H) – while an inpatient at the hospital Warfarin (prior to admission) Other salient information Nuchal rigidity: longstanding, not worse Unsure whether she has had influenza or Hepatitis B vaccine in the past Denies any recent sickness or traveling; denies hemoptysis Born in New York State. Current place of residence is New Hampshire. Has lived in Hawaii when on duty and Kentucky for about one year. Denies history of sarcoidosis or family history of multiple sclerosis Has dogs at home, denies any contact with birds, and denies eating uncooked or raw meats. II. Pertinent findings Clinical VA: OD 20/25-2, OS 20/25-2 (BCVA 3 months previous OD 20/25-, OS 20/20-) PUPILS: ERRL, trace/equivocal APD OS Red cap desaturation: OD 100%, OS 50% EOMS: full, unrestricted, denies worsening of pain on eye movement Confrontation fields: full to finger counting OD, temporal hemianopsia OS Amsler: clear OD, missing temporal field OS up to fixation Color: 11/11 OD, 11/11 OS Page 2 Subjective refraction: OD: +5.75-5.50 x 177, 20/25 OS: +6.00-5.75 x 177, 20/25 Slit lamp examination: unremarkable except for band keratopathy (trace amount) OU, no cells or flare OU Tonometry: OD 14, OS 14 Dilated fundus examination: ( see photo below for OS) OD OS tr – 1+ elongated cells with small heads in anterior and posterior vitreous Vitreous rare cell Margins distinct Rim ONH pink, healthy 0.25 H/V Macula tr pigment changes inferior to macula, no edema, no blood tr pigment changes inferior to macula, no edema, no blood Vessels A/V 2/3 with mild tortuosity, no vasculitis A/V 1:4 (dilated veins) with moderate tortuosity, no vasculitis Posterior Pole clear small (1/16 to ¼ DD) round chorioretinal lesions scattered throughout the arcades and extending to the equator (greatest in number superior temporally and nasally), spares macular area Periphery ¼ DD focal chorioretinal atophy at 8:00 and 9:30 clear OS indistinct 360 with heaping, flame-shape hemorrhage at 7:00, paton’s lines at 2-3:00, deeper intraretinal hemes about ¼ DD away from ONH at 7:00, flame-shaped hemes about ½ DD away from ONH at 10-11:00 elevated 360 no cup Page 3 HVF 30-2: (photos) Normal OD, temporal field cut OS Spectralis OCT: (photos) OS: scan through chorioretinal lesions demonstrates RPE disruption with clumping of questionable material overlying the RPE and below the neurosensory retina FA (done previously through ophthalmology): (photos) OS: window defects exhibiting early and late hyperfluorescence at the level of the RPE corresponding to the gray-yellow punctate lesions in the photographs Note: Images of Humphrey visual field, Spectralis optical coherence tomography, serial fundus photos, and fluorescein angiography are all available and will be included as part of the visuals for this case Physical Dizziness, no vertigo Tingling in peripheral extremities (fingers, toes) Increased fatique Patient report of heart “fluttering” Laboratory studies The patient underwent an intensive workup that included blood and chemistry examinations, magnetic resonance imaging and lumbar puncture. Laboratory studies to assist in ruling out infectious and inflammatory etiologies were obtained. Lumbar puncture with fluoroscopy: normal opening pressure, normal constituents, negative cultures VDRL: negative Angiotensin converting enzyme (ACE): normal MS Panel: Oligoclonal bands: negative result Myelin basic protein: 0.27 (0.00-1.10) OTHER TESTING: ANCA: negative ESR: 43; CRP: 25.90 (RR=0.00-7.43) RPR: non-reactive FTA-ABS: non-reactive Lyme (ELISA): negative RF: negative ANA: negative Anti-double stranded DNA: negative Cardiolipin IgG, IgM: negative Lupus anti-coagulant: negative SSA Antibody: negative SSB Antibody: negative HEP C: negative HEP B: negative Factor V Leiden Homozygous: normal Protein S: 37---ON ANTICOAG (RR=60-140) Protein C: 36---ON ANTICOAG (RR=70-180) Antithrombin III: 96 (RR=80-120) Prothrombin mutation homozygous: normal Aspergillus, coccidioides, blastomyces, histoplasma antibodies: all negative Toxoplasmosis AB IgG, IgM: negative Radiology studies MRI: Scattered white matter lesions not typical of demyelinating disease. More likely due to small vessel disease. Page 4 III. Differential diagnosis The patient’s symptoms and clinical manifestations are suggestive of a “white dot syndrome.” Given the rarity of the white dot syndromes and the extent of disc edema, it was felt prudent to rule-out infectious and inflammatory etiologies that could present similarly. Infectious or inflammatory differentials: Optic neuritis associated with multiple sclerosis (MS): The patient reported various symptoms characteristic of MS (tingling in fingers and toes, longstanding muscle weakness, fatigue); therefore, the chorioretinal lesions and optic neuritis were regarded as separate entities, and a demyelinating disease workup was conducted. Unilateral presentation of increased cranial pressure Note: The above two differentials were considered by ophthalmology prior to the patient presenting to our clinic. In light of the patient’s age and gender and without review of past eye records, it was thought that the disc edema could be presenting in an eye with a past event of chorioretinitis. Presumed ocular histoplasmosis syndrome (POHS): POHS shares similar clinical characteristics with the white dot syndromes. Other infectious/inflammatory etiologies causing similar presentations of either papillitis, chorioretinal inflammation, or both: Sarcoidosis Lyme disease Tuberculosis Syphilis Punctate outer toxoplasmosis Viral infections (i.e., herpes zoster, herpes simplex, Epstein-Barr virus) White dot syndromes: A collection of rare inflammatory disorders affecting the retina, retinal pigmented epithelium, and choroid. Many of these conditions have similar presentations, but also exhibit distinct differences. Although the white dot syndromes are usually associated with white dots, these “dots” only comprise a portion of the clinical presentation. The white dot syndromes include acute posterior mulifocal placoid pigment epitheliopathy (APMPPE), birdshot chorioretinopathy, diffuse unilateral subacute neuroretinitis (DUSN), multiple evanescent white dot syndrome (MEWDS), multifocal choroiditis with panuveitis (MFCPU), and serpiginous choroiditis. In review of the above white dot syndromes, the differential diagnosis was narrowed down based on clinical presentation and findings to the following two white dot syndromes: MEWDS and MFCPU. Multiple Evanescent White Dot Syndrome (MEWDS) MEWDS typically affects myopic females between the ages of 20-45 years of age. The presentation is usually unilateral. Patients typically present with an acute onset of visual alterations including the following: blurred vision, temporal or paracentral scotomas, photopsia and dyschromatopsia. A preceding viral illness is present in about one-third of patients. Visual acuity is variable (ranges from 20/20 to 20/400). A relative afferent pupillary defect may be present. Vitreous cells are mild. The optic disc may edematous and the white dots are usually paramacular in location and fade within the first few weeks of the disease. Visual field testing reveals an enlarged blind spot. Fluorescein angiography demonstrates early and late hyperfluoresence of the white dots. The etiology of MEWDS is unknown and usually undergoes spontaneous resolution without treatment; however, visual field loss or photopsias may persist. Recurrence is unusual. Page 5 Multifocal Choroiditis and Panuveitis (MFCPU) 80% of cases of MFCPU present with bilateral blurry vision, metamorphopsia, floaters, scotoma, and photopsias. Most patients are white, female and myopic. MFCPU shares clinical characteristics similar to presumed ocular histoplasmosis syndrome (POHS); however, unlike POHS, MFCPU demonstrates vitreous inflammation and is not associated with living in an area endemic for histoplasmosis. Punctate inner choroidopathy, another white dot syndrome, is thought to be a variation of MFCPU without vitritis. In addition to intraocular inflammation, patients with MFCPU may have old, punched-out chorioretinal scars or acute yellow-white lesions found in the posterior pole and periphery. These patients may develop choroidal neovascular membrane (25-40%) or cystoid macular edema. Visual field testing may reveal an enlarged blind spot. Fluorescein angiography demonstrates early hypofluoresence and late hyperfluoresence of acute lesions. The etiology of MFPCU is unknown and tends to be chronic and recurring. The active white lesions eventually become chorioretinal scars with pigmented borders and visual loss is common secondary to choroidal neovascular membrane formation. Page 6 IV. Diagnosis and discussion Given the unrevealing laboratory and imaging studies, the focus in diagnosis was turned to the “white dot syndromes.” The question that remained unanswered was which white dot syndrome did this patient actually have? The white dot syndromes all predominantly affect young to middle-aged patients, specifically females. The diagnostic dilemma lies in the overlap among the various syndromes as well as the lack of understanding of the etiology or pathogenesis in the presentation of findings. While some suggest that the white dot syndromes represent a continuum of clinical presentations, others argue that each white dot syndrome is a distinctive entity. This case presentation was unusual in that a major finding was the striking optic disc edema. Optic disc edema, although associated with many of the white dot syndromes, is not typically as fulminant as in our case. In addition, the patient was moderately high hyperope, not myopic as is the case in most white dot presentations. In examining all the different characteristics, the diagnosis was narrowed, but not limited to, two different white dot syndromes – namely, multifocal choroiditis with panuveitis (MFCPU) and multiple evanescent white dot syndrome (MEWDS). These two syndromes demonstrate many similar clinical characteristics including the following: predominantly affect young, myopic females and are associated with onset of photopsias, metamorphopsias, decreased acuity, visual field defect (most notably an enlarged blind spot), and vitreal cells. There are a couple characteristics that differ with MFCPU and MEWDS. For one, the appearance of the white dots tends to be slightly different; although, the white dots are similar sizes, the dots in MFCPU have been found to vary more in shape and size and are found to be greater out toward the periphery. In MEWDS, the white dots tend to take on a paramacular distribution. In our patient, the white dots were more extramacular and greater along the superior temporal arcades and nasal to the optic nerve head. In addition, the white dots in MFCPU tend to scar and remain following an episode while in MEWDS, the dots resolve and slowly disappear. Slight resolution was noted in our patient at her last visit which may suggest that her presentation is more consistent with MEWDS. The fluorescein angiogram in this patient exhibited hyperfluoresence in both early and late phases. This is more characteristic of MEWDS. MFCPU tends to exhibit early hypofluroesence and late hyperfluoresence in acute lesions. Although not extensively studied, optical coherence tomography may show slight differences between MEWDS and MFCPU; however, more research in this area is necessary. MEWDS tends to demonstrate photoreceptor inner segment/outer segment disturbances while MFCPU exhibits a clumping of material above the level of the retinal pigmented epithelium – more consistent with that found in our patient. As the case evolves, the diagnosis should become clearer. Currently, MEWDS is the favored diagnosis. Page 7 V. Treatment, management The big question with many conditions is to or not to treat. MEWDS tends to be self-limiting where as MFCPU tends to demonstrate recurrences and a poorer visual prognosis. Most cases of MEWDS tend to exhibit only mild disc edema which resolves along with the chorioretinal lesions. In one study of MFCPU with optic disc edema, oral corticosteroids demonstrated a decreased recurrence rate and better visual outcomes. Our case is still evolving. At this point in time, no treatment has been initiated; however, corticosteroid treatment is being considered. Page 8 VI. Conclusion Our patient has multiple systemic and psychological issues which supported the need for a full workup to rule out inflammatory and infectious etiologies that may present similarly. The lack of definitive diagnosis and, therefore, the uncertainty of prognosis is frustrating for the patient and doctor alike. Thorough assessment to rule-out treatable conditions, extensive patient education, and close follow-up with reconstitution of intervention as the case evolves are the key to an optimal outcome. Page 9 Bibliography De Meyer , V., et al. “Multifocal Choroiditis with Panuveitis and Punctate Inner Choroidopathy: A Mini Review.” Bull. Soc. Belge Ophthalmol 1999: 115-124. Dreyer, Richard F., and Donald M. Gass. “Multifocal Choroiditis and Panuveitis: A Syndrome That Mimics Ocular Histoplasmosis.” Arch Ophthalmol December 1984; 102: 1776-1784. Goldstein, Debra A., and Larry Ulanski. “Multifocal Choroiditis vs. PIC: Variations on a Theme?” Review of Ophthalmology 2004; 11(6). Gray, R.H. “Unilateral enlargement of the blindspot: a diagnostic dilemma.” www.bjophthalmol.com (July 17, 2009). Jampol, Lee M. and Amanda Wiredu. “MEWDS, MFC, PIC, AMN, AIBSE, and AZOOR: One Disease or Many”? Retina 1995; 15 (5): 373-378. Kaiser, Friedman, et al. (2004). The Massachusetts Eye and Ear Infirmary Illustrated Manual of Ophthalmology. 2nd edition. Philadelphia: Elsevier Science. Kanski, J. J. (2007). Clinical Ophthalmology, 6th Ed. Butterworth-Heinemann. Matsumoto, Yoko , Sebastian Haen, and Richard Spaide. “The White Dot Syndromes.” Medscape.com (July 17, 2009). Mansour, S., et al. “Multifocal Choroidopathy Syndromes.” Emedicine from WebMD (July 19, 2009). Nguyen, My Hanh T., et al. “Microstructual abnormalities in MEWDS demonstrated by ultrahigh resolution optical coherence tomography.” Retina 2007: 27 (4): 414-418. Parenell, J.R., et al. “Differentiation Between Presumed Ocular Histoplasmosis Syndrome and Multifocal Choroiditis with Panuveitis Based on Morphology of Photgraphed Fundus Lesions and Fluorescein Angiography.” Archives of Ophthalmology February 2001: 19: 208-212. Quillen, David A., et al. “Perspective: The White Dot Syndromes.” American Journal of Ophthalmology March 2004: 538-550. Shenoy, Radha, and Ahmed Al Hinai. “Presumed Ocular Toxoplasmosis Presenting as Papillitis.” Indian Journal of Ophthalmology 2003; 51: 357-59. Tewari A, Elliot D. “White Dot Syndromes.” 2007. Emedicine from WebMD (July 17, 2009). Thorne, Jennifer E., et al. “Multifocal Choroiditis with Panuveitis: Incidence of Ocular Complications and of Loss of Visual Acuity.” Ophthalmology December 2006: 113 (12): 2310-2316. Page 10 Thorne, Jennifer E., et al. “Optic neuropathy complicating multifocal choroiditis and panuveitis (MFCPU).” American Journal of Ophthalmology April 2007; 143 (4): 721-723. Vianna, Raul N.G., et al. “Longterm follow-up of patients with multifocal choroiditis and panuveitis.” Acta Ophthalmologica Scandinavica 2004: 748-753. Volpe, Nicholas J., et al. “Acute Idiopathic Blind Spot Enlargement Syndrome: A Review of 27 New Cases.” Archives of Ophthalmology January 2001; 119: 59-63. Page 11