* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Isomerisms
Biosynthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Butyric acid wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
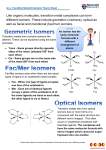
L.S.T. Leung Chik Wai Memorial School F.6 Chemistry Chapter 27: Isomerism 同分異構體 Chpt.26: p.1 ISOMERISM OF ORGANIC MOLECULES 同分異構 Introduction Isomerism is the existence of different compounds with same molecular formaulae 分子式 but different structural formulae結構式.(arrangement of atoms). Note : <1> <2> <3> II. Those different structures with the same molecular formula are called isomers. Isomers have different physical and chemical properties. There are two types of isomerism in general structural isomerism 結構異構and stereoisomerism 立體異構. Structural Isomerism Structural isomers are compounds which have identical molecular formual but differ in the order in which the atoms are joined together. (A) (B) Chain isomerism 鏈異構 The isomers have different carbon chain. (different arrangement of carbon atoms in the molecule. Example: Molecular formula C5H12 pentane 2-methylbutane 2,2-dimethylpropane 戊烷 2-甲基丁烷 2,2-二甲基丙烷 Positional isomerism 位置異構 These isomers have a substituent group in different position in the same carbon ‘skeleton”. Examples (i) propan-1-ol and propan-2-ol 丙-1-醇 丙-2-醇 (ii) (iii) 1—methoxypropane 1-甲氧基丙烷 1 ,2—dibromobenzene and 2—methoxypropane 2-甲氧基丙烷 and 1, 3—dihromobenzene L.S.T. Leung Chik Wai Memorial School F.6 Chemistry Chapter 27: Isomerism 同分異構體 (C) Chpt.26: p.2 Functional group Isomerism 官能基異構 These isomers have different functional groups and belong to different homologous series. Examples (i) An alcohol and an ether (e.g. C2H6O) methoxymethane 甲氧基甲烷 (ii) ethanol 乙醇 An aldehyde and ketone (C3H6O) propanone 丙酮 (iii) propanal 丙醛 A carboxylic acid and one or more esters. butanoic acid 丁酸 Exercise 1 Draw the possible isomers of (a) C4H6 methyl propanoate 甲酸丙酯 (b) C3H6O [Hint : Formula for degree of unsaturation = no. of C +1 - H X N 2 2 2 where C, H, X and N are the number of atoms respectively] SOLUTI ON Exercise 2 A research worker reported that there are six possible would be dichlorobenzoic acid, C6H3Cl2COOH which would be decarboxylated to dichloroenzenes, C6H4Cl2. One of the six, three isomeric acids gave the same dichlorobenzene X, two gave a dichlorobenzene Y and one acid gave another dichlorobenzene Z. Draw the appropriate structures for each isomeric dichlorobenzene, labelling each correctly as X. Y and Z. Then give the structures of. the dichlorobenzoic acids from which each is derived. SOLUTI ON L.S.T. Leung Chik Wai Memorial School F.6 Chemistry Chapter 27: Isomerism 同分異構體 III. Chpt.26: p.3 Stereoisomerism立體異構體 Stereoisomers have different spatial arrangement (atoms are arranged differently in space). Stereoisomerism can be divided into two categories geometrical isomerism幾何異構 and optical isomerism旋光異構. (A) Geometrical Isomerism 幾何異構體(cis順 / trans反) Geometrical isomers are stereoisomers for which the spatial arrangement of the atoms is different because of restricted rotation about a covalent bond. Examples : cis-but-2-ene 順-丁-2-烯 <1> trans-but-2-ene反-丁-2-烯 <2> cis-1 .2-dibromoethene 順-1,2-二溴乙烯 trans-1, 2-dibromoethene 反-1,2-二溴乙烯 Note: <1> <2> Cis- means “on the same sides” 在同一側whereas trans- means “on the opposite sides” 在相反一側 Geometrical isomerism sometimes known as is “cis-trans isomerism”. Trans compounds have usually higher stability because the bulkier groups are further apart. Such isomer has lower intrinsic energy. <3> Possibility for geometrical isomers C A C B C A C C D B C D Where AB and CD Determine which of the following would have stereoisomerism ____________ ____________ _____________ ____________ Example : cis-butenedioic acid 順-丁烯二酸 and trans-butenedioic acid反-丁烯二酸 Exercise 3 (a) Write all structural formulae of C5H10 which contain a double bond. (b) Point out which structures in (a) can exhibit geometrical isomers. SOLUTION L.S.T. Leung Chik Wai Memorial School F.6 Chemistry Chapter 27: Isomerism 同分異構體 Chpt.26: p.4 Properties of Geometrical Isomerism (A) Physical properties (e.g. melting point and boiling point) Consider the following difference in melting and boiling points of two pairs of geometrical isomers Explanation <1> Trans-isomers usually have higher melting points. Trans-isomers have bulky groups 巨 形基組on either side of the double bond to give a more symmetrical structure 對稱結 構 so that they can pack more compactly有效裝填 in their solid state molecular crystals. As a result, the intermolecular forces between the trans-isomers become higher. <2> Cis-isomers usually have higher boiling points. In cis—isomers, both CH3— and Cl— groups exert inductive effects on the double bond. The result would give rise a net dipole of the molecule. So the additional dipole attraction between cis—isomers causes them to have higher boiling points. Note : Some geometrical isomers have even more drastic difference in their physical properties and chemical properties. Example : Cis—butenedioic acid and trans—butenedioic acid Cis—butenedioic acid has a melting point of 130 0C whereas that of trans—butenedioic acid is 290°C. Explanation : The intermolecular hydrogen bonds分子間的氫鍵 for cis— butenedioic acid are less extensive than those of trans—isomers. <1> L.S.T. Leung Chik Wai Memorial School F.6 Chemistry Chapter 27: Isomerism 同分異構體 <2> Chpt.26: p.5 The dehydration of the acid The cis—isomer undergo dehydration to give an acid anhydride at 150 0C whereas the trans—isomer can only do when the temperature is at 250°C. Explanation: The two carboxylic groups in cis—isomers are on the side so that it is easier to under dehydration to give acid anhydride. However, higher temperature is required to cause the bond between the carbon atoms for the trans—isomers to give the cis—isomers which can subsequently undergo dehydration. The butenedioic anhydride can be redissolved in boiling water to form a solution of cis—butenedioic acid; <3> More comparisons between the cis— and trans— acids When conc. HClis added to the solution of cis—butenedioic acid and the mixture refluxed for about 20 minutes, hydrogen chloride adds onto the cis—butenedioic acid molecule to form an intermediate in which there is unrestricted rotation about the C—C bond. Crystals of the trans—isomer separate out when the resulting solution is cooled in a cold water bath or an ice bath. They can be removed by suction filtration and washed by cold water and then dried. L.S.T. Leung Chik Wai Memorial School F.6 Chemistry Chapter 27: Isomerism 同分異構體 Chpt.26: p.6 Comparison of the acid strength: The cis- and trans- isomer can furnish hydrogen ions in water, making the resulting solution weakly acidic: However, the cis— isomer is much more acidic than the trans— isomer, as can be seen in their acid dissociation constants. (1) For K1 cis— acid > trans— acid -For K2 : cis— acid < trans— acid Reason : For cis— acid, the formation of intramolecular hydrogen bond分子內氫鍵 draws electron density away from the other O—H group, weakening its bond and making the release of the first proton much more readily. Also, after the first proton is lost the residual charge on the ion exhibit special stability. This make the K2 value lower than that of the trans— acid. Table 2 Specimen results of investigation of some properties of cis- and trans- butenedioic acids 1. 2. Water solubility: cis acid is more soluble Melting point of cis- acid: 130 °C Melting point of trans- acid: 287 °C (sublimes) 3. Acid strength Test cis-acid pH of solution 1.6 (0.04 mol dm3) action with Mg Mg (s) dissolved readily with evolution of H2 (g) Reaction with Na2CO3 Effervescence of CO2 (g) more readily 泡騰現象 4. trans-acid 2.7 Reacts much less readily Reaction with bromine water. Decolorization of bromine water occurs readily when the acid (cis- or trans) is in excess. L.S.T. Leung Chik Wai Memorial School F.6 Chemistry Chapter 27: Isomerism 同分異構體 Chpt.26: p.7 (B) Optical Isomerism Any carbon atom (sp3 hybridization雜化) with four different atoms or groups attached to it is asymmetric不對稱. Example : The central carbon atom of 2—hydroxypropanoic acid is asymmetric. No matter how the molecule is rotated, twisted or turned, it cannot be superimposed (不能重疊) on its mirror image 鏡像. The two molecules, which are mirror images of each other, are thus isomers. They are called enantiomers 對映異構體. Note : optical <1> Any Carbon atoms attach with Four different group of atoms is called chiral carbon手徵性碳原子. Those compounds with chiral carbon is called chiral molecules 手徵性分子. <2> Enantiomers may exist separately or as mixtures. A mixture containing equal numbers of moles of each enantiomers is known as racemic mixture 消旋混合物. Separation of a racemic mixture into two pure enantiomers is known as resolution. <3> A pair of enantiomers are chemically and physically identical with each other in almost every respect except for one vital重要difference. This difference is their activity 旋光性. L.S.T. Leung Chik Wai Memorial School F.6 Chemistry Chapter 27: Isomerism 同分異構體 1. Chpt.26: p.8 Properties of Enantiomers : Optical Activity 旋光性 The waves of a ray of normal light vibrate in all directions at right angles to the direction in which the ray is travelling. Light which vibrates in one plane only is called plane—polarized light 偏振光. A compound which can rotate plane-polarised light so that the light vibrates in a different plane is said to be optically active. In order that a compound be optically active, its molecules (or ions) must be asymmetric. All compounds which contain a single asymmetric carbon atom exhibit optical activity. All enantiomers exhibit optical activity and are thus called optical isomers. One enantiomer rotates a plane of light clockwise whereas the other rotate it anticlockwise. 右旋 左旋 L.S.T. Leung Chik Wai Memorial School F.6 Chemistry Chapter 27: Isomerism 同分異構體 Chpt.26: p.9 Note : <1> Maximum number of optical isomers = 2n where n is the no. of chiral carbon. <2> Optical isomerism is important in biological systems. For example, the amino acids used in the construction of proteins are all optically active with few exception. e.g. 2-aminopropanoic acid 2-胺基丙酸(alanine) The Polarimeter 偏振計 A polarirneter is a device for measuring the effect of plane polarised light on optically active compounds. The angle through which an enantiomer rotate plane-polarised light is specific for that enantiomer. The angle can be measured using a polarimeter. Measuring procedure <1> <2> <3> <4> A unpolarised monochromatic light (consists of a single wavelength only. e.g. light form a sodium lamp) is pass through a polariser which converts it to plane-polarised light. The plane-polarised light is then passes through a tube containing a solution of the sample whose angle of rotation is to be measured. On emerging from the sample tube, the plane polarised light has been rotated either clockwise or anticlockwise through the angle to be measured. The direction of rotation is defined with respect to the observer and measured in a polarimeter. The angle is measured using an analyzer which can only allows plane-polarised light to pass through it. It is initially set in line with the plane-polarised light transmitted by the polariser before it is rotated by the sample. Plane-polarised light rotated by the sample cannot pass through the analyzer when it has this initial setting. The analyzer is then slowly rotated until the rotated light can pass through. At this point its transmission plane is in line with that of the rotated plane-polarized light. The angle at this point is measured. Specific Rotation 旋光率 []tD = lc Where l is the length of the sample tube (in 1 dm), c is the concentration of sample (in g per cm3 ) D is D-line of sodium lamp t is temperature 250C L.S.T. Leung Chik Wai Memorial School F.6 Chemistry Chapter 27: Isomerism 同分異構體 Chpt.26: p.10 Exercise 4 A chloroalkyne is found to be optically active. When it absorbs one mole of hydrogen to become a chloroalkene, it is still optically active. However, when it absorbs a further mole of hydrogen, the resulting saturated chloroalkane is optically active. Deduce the structure of the chloroalkane, if it is found to have a molecular formula of C5H7Cl. SOLUTION Exercise 5 (a) What is meant by the term optical activity? (b) What conditions must be fulfilled in order that a compound may exhibit optical activity? (c) What are the essential requirements for a structure to exhibit geometrical (cis—trans) isomerism? (d) Indicate which of the following structures may exhibit stereoisomerism, and, where they do, draw a diagram of each stereoisomer. You should indicate clearly what type(s) of stereoisomerism is(are) involved. CH3CH2CH2CH(OH) CH3 CH3CH2CH(OH)CH2CH3 CH3CH=CHCOOCH(CH3)C6H5 L.S.T. Leung Chik Wai Memorial School F.6 Chemistry Chapter 27: Isomerism 同分異構體 Chpt.26: p.11 Exercise 6 Give explanation, the possible structure(s) for the compounds X and Y respectively. (a) X is a sweet smelling liquid which contains 58.82% carbon, 9.80% hydrogen and 31.37% oxygen and has relative molecular mass between 90 and 110. It is also found that X is optically active. (b) Y, C5H9Br, is an acyclic bromoalkene which is optically active. SOLUTION Exercise 7: Explain how would you confirm whether a molecule has an optical rotation of +600 or –3000 . SOLUTION: Dilute the solution measured 4times, if the original rotation is +600, the new rotation should be +15°; if the original rotation is -3000, the new rotation should be -75°.