* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download MechDrug Interact_07..
Survey
Document related concepts
Transcript
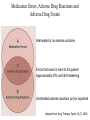
Mechanisms (and some consequences) of Drug Interactions September 18, 2007 Frank F. Vincenzi Learning Objectives • • • • Interaction Object Drug Precipitant Drug Pharmacological Antagonism • Physiological Antagonism • Pharmacokinetic Interaction • Pharmacodynamic Interaction • Additive Effects • Synergism (potentiation) • Adverse Drug Event • Adverse Drug Reaction Definitions • The effects of an OBJECT DRUG are altered by the presence of another drug - the PRECIPITANT DRUG • Operational Classifications of Drug Interactions (Hansten & Horn) – Class 1: Avoid (Risk >> benefit) – Class 2: Usually avoid (alternatives or if benefit > risk) – Class 3: Minimize risk (consider alternatives, circumvent, monitor – Class 4: No special precautions (risk << benefit) – Class 5: No evidence of interaction Definitions • The effects of an OBJECT DRUG are altered by the presence of another drug - the PRECIPITANT DRUG • Operational Classifications of Drug Interactions (ePocrates) – – – – Contraindicated Avoid/use alternative Monitor/modify treatment Caution advised A potentially lifesaving pharmacodynamic drug interaction: In a classic case of competitive pharmacological antagonism, the respiratory depressant effects of the opiate agonist, heroin, are antagonized by the opiate antagonist, naloxone (Narcan@). A potentially lifesaving pharmacodynamic drug interaction Physiological antagonism bronchoconstriction associated with anaphylaxis (e.g., penicillin allergy) is antagonized by epinephrine. Histamine and other mediators act on certain receptor(s) to cause bronchoconstriction. Epinephrine acts on different receptors to exert an opposite effect on the physiological system. Example of potentially lethal pharmacodynamic drug interaction (Class 1: Avoid Interaction) • Amiodarone (Cordarone®) increases QTc interval (ECG - QT interval corrected for heart rate) at therapeutic concentrations • So do: – – – – – Disopyramide (Norpace®) Procainamide (Pronestyl®) Propafenone (Rhythmol) Quinidine (Quinidex®) Sotalol (Betapace®) Example of potentially lethal pharmacodynamic drug interaction (Class 1: Avoid Interaction) • Selegeline (Eldepryl®) (MAO inhibitor) • Among others, avoid: – – – – – Alpha agonists (all) Opiates (all) Selective serotonin reuptake inhibitors (all) Tricyclic antidepressants (all) Yohimbine Example of potentially toxic or lethal pharmacodynamic drug interaction (Class 3: Caution advised, monitor) • Morphine depresses respiration. Synergistic depression of respiration may occur with: • • • • • • • • Anticholinergics Antidepressants Antihistamines Antipsychotics Barbiturates Benzodiazepines Ethanol Muscle relaxants Case: Wm. Unterexerciser • 42 year old obese accountant; diagnosed with sleep apnea after his wife complained of excessive snoring • Following surgical repair patient was monitored with an oximeter in the recovery room • In hospital room (no oximeter), several injections of meperidine (Demerol®) gave little pain relief • Injection of 10 mg of diazepam (Valium®) IV provided relief and the patient went to sleep • Several hours later the patient was found dead William Osler was right! • “The disease the patient has is less important than the patient that has the disease” • Some patients with sleep apnea have become chronically desensitized to the accumulation of carbon dioxide • Opioids depress respiratory drive in part by decreasing the sensitivity of the brain to the accumulation of carbon dioxide • A number of sleep apnea patients have died after surgical repair when treated with opioids postoperatively. Pharmacokinetic (dispositional) drug interaction: changing the amount of target drug at its receptors by altered drug absorption • Binding drug in GI tract – Antacids – Cholestyramine – Sucralfate • Modification of GI pH – Proton pump inhibitors, H2 antagonists, antacids • Greatly reduce absorption of itraconazole or ketoconazole • Induction or inhibition of P-glycoprotein (PGP) Quinidine or verapamil inhibit PGP and increase the absorption of digoxin. Pharmacokinetic (dispositional) drug interactions: altered drug abs/distribution and P-glycoprotein • PGP: luminal membrane of epithelial cells in the gut, blood-brain barrier kidney & placenta - also some bacterial & cancer cells. PGP limits absorption from the gut, access to the CNS, promotes secretion of drugs in kidney tubules & protects the fetus. • Substrates of PGP are many (lipid sol.). Some examples: – Daunorubicin, digoxin, fexofenadine, paclitaxel, rifampin, verapamil, vinblastine & vincristine • Inhibitors of PGP on our drug list include: – Amiodarone*, diltiazem, erythromycin, indinavir, itraconazole, ketoconazole*, quinidine & verapamil • Inducers of PGP include: – Rifampin, St. John’s wort P-glycoprotein is a product of the multidrug resistance gene (MDR) Pharmacokinetic (dispositional) drug interactions: changing the amount of target drug at its receptors by altered drug elimination • Displacement from protein binding – Increases glomerular filtration of small molecules • Modification of tubular urine pH – Sodium bicarbonate, ammonium chloride, acetazolamide • Induction or inhibition of P-glycoprotein (PGP) – May change the amount of drug actively secreted by the kidney tubules (see previous slide) Pharmacokinetic (dispositional) drug interactions: changing the amount of target drug at its receptors by altered drug metabolism • Theophylline (metabolized by CYP1A2 > CYP3A4) – Most quinolone antibiotics inhibit 1A2) • Ciprofloxacin (Cipro®) – NOTE: Levofloxacin has little effect on CYP – Most macrolide antibiotics inhibit 3A4 • Erythromycin (E-Mycin®) – NOTE: azithromycin [Zithromax®] does not inhibit CYP3A4 • (Class 2: Use only when benefits > risks) Effect of a component of grapefruit juice on felodipine pharmacokinetics OJ (solid), GJ (triangle), furanocoumarin-free GJ (square) Paine et al., 2006 Medication Errors, Adverse Drug Reactions and Adverse Drug Events Intercepted or no adverse outcome Errors that result in harm to the patient Approximately 20% are life-threatening Unintended adverse reactions (un)/or expected Adapted from Drug Therapy Topics 34 (7), 2005 Medication Errors: One Example • 106 cases of medication errors associated with methotrexate were identified, including errors resulting in deaths (24%) and other serious outcomes (45%). • The most common types of errors involved confusion about the once-weekly dosage schedule (30%) and other dosage errors (22%). • Of the errors, 39 (37%) were attributable to the prescriber, 21 (20%) to the patient, 20 (19%) to dispensing, and 18 (17%) to administration by a health care professional. Moore et al., 2004, Medication Errors Associated With Methotrexate, American Journal of Health-System Pharmacy Reported SALAD (Sound-Alike Look-Alike Drugs) Pharmacist reported a potential error involving confusion between INSPRA® (eplerenone), a selective aldosterone receptor antagonist, and SPIRIVA® (tiotropium), an inhalation powder indicated for bronchospasm associated with COPD. Physician ordered Spiriva® 25 mg PO daily. Since Spiriva® is a powder for inhalation and shouldn't be swallowed, the pharmacist called the physician who verified intention was for Inspra® 25 mg PO daily. Another SALAD Drug Example Fluvoxamine (Luvox®)100 mg, was stocked in an automated dispensing cabinet instead of flavoxate (URISPAS® ) 100 mg. The hospital is now labeling these medications as fluvoxAMINE and flavoxATE. One source of medication error now avoided • Effective June 7, 2006 Washington State law requires all prescriptions to be hand printed, typewritten or electronically generated. • • Rxs in cursive are considered illegible A note from official labeling of a new drug • PALLADONE (hydromorphone extended-release) comes in 12, 16, 24, and 32 mg capsules. This drug should only be used in patients who are already receiving opioid therapy and who require a total daily dose of at least 12 mg of oral hydromorphone or its equivalent. Palladone is given daily but should NEVER be prescribed using the abbreviation "Q.D." It is predictable that if "Q.D." is misread as QID, the 4-fold overdose of this potent narcotic could prove fatal to some patients. Limiting resident work hours does not affect ADE rates • Before or after weekly hours were limited to 80 hours per week there were no significant differences in: – Confirmed ADEs per 1000 patient days – Preventable ADEs Mycyk, Am J Health Syst Pharm 62(15):1592-1595, 2005 Some causes of preventable ADEs • Drug interactions (4.6%) • Excessive dosage (42%) • Known drug allergies (1.5%) • Patient identification errors (3.5%) Adapted from Drug Therapy Topics 34 (7), 2005 Community Pharmacy Drug Interaction Software • Identification of clinically relevant drug-drug interactions (DDIs) • Chain and HMO pharmacies in WA State (~half) • Sensitivity, specificity and positive and negative predictive values in detecting 16 well-established DDIs in six fictitious patient profiles – – – – Sens (correctly identify DDI) [true pos/true pos+false neg]0.44-0.88 Spec (ignore non DDI) [true neg/true neg+false pos) 0.71-1.00 Pos pred value [true pos/true pos+false pos] 0.67-1.00 Neg pred value [true neg/true neg+false neg] 0.69-0.90 • Same software gave different results in different locations • Failed to detect clinically relevant DDIs one-third of the time • Suboptimal performance - not really reliable Hazlet et al. J Am Pharm Assoc 41(2):200-204, 2001 PDA Software for Drug Interactions? • Sensitivity, specificity and pos and neg predictive values, speed to identify five important drug interactions, ease of use • Possible 800 points – – – – – iFacts 777 Lexi-interact Mosby’s Drug Consult 688 Mobile Micromedex 655 ePocrates 559 Barrons, Am J Health-Syst Pharm 61(4) 380-385, 2004 In-Hospital Errors • July 27, 2004 • Nearly 195,000 people in the U.S. died each year as a result of potentially avoidable medical errors in 2000, 2001, and 2002, according to a new study of 37 million patient records released today. • "The equivalent of 390 jumbo jets full of people are dying each year due to likely preventable, in-hospital medical errors. • http://www.ghi.com/yourhealth/articles/102228.html • If the CDC's annual list of leading causes of death included medical errors, it would show up as number six, ahead of diabetes, pneumonia, Alzheimer's disease and renal disease. Medication errors • “The greatest single systemic factor associated with medication errors is a deficiency in the knowledge requisite to the safe use of drugs.” Peth, H.A., Medication errors in the emergency department. A systems approach to minimizing risk, Emerg.Med.Clin.N.Am. 21: 141-158, 2003 Drug Classes Associated with >= 5% Adverse Drug Events Gurwitz et al., Am.J.Med., 118: 251-258, 2005 Adverse Drug Events in a Recent Study Gurwitz et al., Am.J.Med., 118: 251-258, 2005