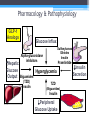
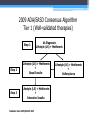
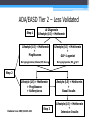
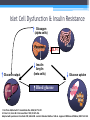
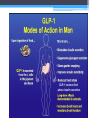
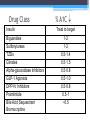
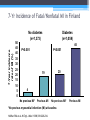
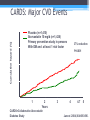
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download beta cells
Survey
Document related concepts
Transcript
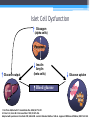
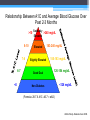
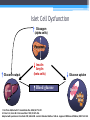
Diabetes Rx: A Primer Laura Shane-McWhorter, PharmD, BCPS, FASCP, CDE, BC-ADM Professor (Clinical) University of Utah College of Pharmacy Department of Pharmacotherapy Objectives • Describe presentation differences in persons with Type 1 and Type 2 diabetes • Explain initial drug therapy choices for persons with Type 2 diabetes • Differentiate between the available drug classes for treatment of Type 2 diabetes, based on dose, ADRs, pharmacokinetics, and efficacy • Given a patient with Type 2 diabetes, develop a monitoring plan, including labs for disease outcomes and drug-related monitoring Diabetes Mellitus (DM) A chronic disorder: • Characterized by hyperglycemia • Abnormal CHO, fat, protein metabolism • Acute complications (hypo/hyperglycemia, secondary infections) • Marked propensity to develop chronic complications: • Renal Microvascular • Ophthalmic • Neurologic • Cardiovascular disease - Macrovascular Diabetes: The Statistics • • • • Persons with diabetes: • 24 million persons • 12.2 million in > 60 yrs 1.6 million new cases • diagnosed in people aged 20 years or older in 2007 Pre-diabetes: 57 million people Lifetime risk • • • Males: 32.8% Females: 38.5% Hispanic women: 52.5% www.diabetes.org/diabetes-statistics/prevalence.jsp Prevalence of Diabetes in the USA Diagnosed Diabetes 17.5 Million Undiagnosed Diabetes 6.6 Million Diabetes Costs • 2007 Costs of diabetes in the US: • Total: $174 billion • Medical costs • $116 billion • Decreased productivity (absenteeism, work productivity, inability to work due to disability, and premature mortality) • $58 billion Diabetes Care 2008;31:1-20. Diabetes Costs • 2008 Costs of diabetes in the US: • Total: $218 billion (10% of USA healthcare spending) • Medical costs - $174.4 billion • $14.9 billion for T1DM • $159.5 billion for T2DM • Cost for undiagnosed DM - $18 billion • Cost for pre-DM - $25 billion • Cost for GDM - $636 million Associated Press Diabetes Statistics… • Medications/supplies • $3.7 billion for insulin • $1.8 for supplies • $8.6 billion for oral agents • $12.7 billion for retail Rxs Diabetes Care 2008;31:1-20. BJ • BJ is a 20 y/o junior in college. She is concerned about having a lot of UTIs in the last eight months. She is seen at Student Health for an upper respiratory infection and random glucose values in the last month have been > 200 mg/dL. She complains of polyuria and polydipsia. She has also been losing weight without trying. Her labs are the following: • • • • • Glucose 340 mg/dL + Glutamic Acid Decarboxylase Antibodies C-peptide 0.5 ng/mL (0.5-5 ng/mL) + ketonuria 5’4” tall and 104 lbs (weight was 118 lbs 3 months ago) RE • RE is a 45 y/o male seen in clinic for balanitis and onychomycosis. The patient is 5’10” and weighs 240 lb. He complains of thirst and polyuria. His fasting glucose values have been in the low 120s (mg/dL). He has gained 40 lbs in the last 2 years. • Today, random glucose is 359 mg/dL • BP 148/98 mm Hg • Fasting lipids total cholesterol 240 mg/dL, triglycerides 438 mg/dL, HDL of 32 mg/dL Criteria for Diagnosis • Fasting plasma glucose (FPG) > 126mg/dL† • Symptoms of diabetes plus casual plasma glucose concentration > 200 mg/dL* (3 Ps, wt loss) • 2 hr PG during Oral Glucose Tolerance Test (75 g OGTT) is > 200 mg/dL • A1C > 6.5% (NGSP) * Casual is defined as any time of day without regard to time since last meal. † Fasting is defined as no caloric intake for at least 8 hours. • In absence of unequivocal hyperglycemia, confirm by testing on different day (same or different test) • OGTT not for routine use Classification TYPE 1 • • • • • • • • • • • < 30 y/o (75% < 18 y/o) Abrupt onset (wt , 3 Ps) 5-10% FH – emerging genetic basis No insulin production Normal/underweight Ketosis common Whites: more common Etiology: Autoimmune Initially, no microvascular complications Initially, macrovascular complications rare TYPE 2 • • • • • • • • • • • Any age; with age Gradual onset (+ Sx) 90-95% FH – strong Insulin resistance, impaired insulin secretion (may need insulin) 80% overweight Ketosis rare; HHS may occur Ethnic minorities: common Etiology: Obesity? Insulin resistance? Initially microvascular complications common Initially, macrovascular complications common Pathophysiology of Type 1 DM • Primary defect is absolute insulin deficiency with almost total loss of functional beta cell mass in months before diagnosis • Beta cell mass loss usually related to autoimmune destruction of pancreatic beta cells • Fasting hyperglycemia when 80-90% of beta cell mass is destroyed • e.g., no insulin secretion Pathophysiology of Type 1 DM • Measurable antibodies due to autoimmune destruction of beta cells • Glutamic acid decarboxylase autoantibodies (GAD) • Insulin autoantibodies (against islet tyrosine phosphatase) • Islet cell antibodies (not standardized in labs) • Significant HLA association (DR3, DR4) on Chromosome 6 (40 known genes on Chromosome 6 contribute risk) • Strong genetic linkage to DQA and B genes Pathophysiology of Type 1 DM • Disturbances in lipid and amino acid metabolism in those that later declare with T1DM • succinic acid/phosphatidylcholine at birth • TGs/antioxidant ether phospholipids • lysophosphatidylcholines (pro-inflammatory) months before beta cell autoimmunity • Absolute amylin deficiency (co-stored, co-secreted with insulin) • Disrupted compensatory systems of glucose regulation (glucagon) that risk for hypoglycemia and erratic glucose control Presentation of Type 1 DM • 20-40% of T1DM present with DKA after several days of polyuria, polydipsia, polyphagia, and weight loss • Some T1DM pts may enter “honeymoon” phase • Some residual beta cell function Islet Cell Dysfunction Glucagon (alpha cells) Pancreas Glucose output Liver Insulin Amylin (beta cells) Blood glucose Glucose uptake Muscle Adipose tissue 1. Del Prato S,Marchetti P. Horm Metab Res. 2004;36:775–781. 2. Porte D Jr, Kahn SE. Clin Invest Med. 1995;18:247–254. Adapted with permission from Kahn CR, Saltiel AR. Joslin’s Diabetes Mellitus. 14th ed. Lippincott Williams & Wilkins; 2005:145–168. BJ • BJ is a 20 y/o junior in college. She is concerned about having a lot of UTIs in the last eight months. She is seen at Student Health for an upper respiratory infection and random glucose values in the last month have been > 200 mg/dL. She complains of polyuria and polydipsia. She has also been losing weight without trying. Her labs are the following: • • • • • Glucose 340 mg/dL + Glutamic Acid Decarboxylase Antibodies C-peptide 0.5 ng/mL (0.5-5 ng/mL) + ketonuria 5’4” tall and 104 lbs (weight was 118 lbs 3 months ago) BJ • How should we confirm the diagnosis of DM when a glucose is repeated? • • • • Fasting glucose? Postprandial glucose? OGTT? A1C? BJ • What tests suggest that BJ has Type 1 DM? • • • • • Glucose 340 mg/dL? + Glutamic Acid Decarboxylase Antibodies? C-peptide 0.5 ng/mL (0.5-5 ng/mL)? + ketonuria? 5’4” tall and 104 lbs (weight was 118 lbs 3 months ago)? What is A1C and Why is it Important ? • Glucose attaches to proteins throughout the body through a reaction called glycosylation • HbA is the predominant form of Hb contained in RBCs • This serves as a marker for the extent of protein glycosylation • HbA has 3 fractions (1a, 1b, 1c) where 1c is the predominant form (95%) • The higher the BG the greater the fraction of A1C that is glycosylated • A1C represents average BG over previous 3 months • Normal A1C is 4-6% (<126 mg/dL) • Pre-DM: 5.7-6.4% ADAG Trial • Average glucose (mg/dL) • 28.7 x A1C – 46.7 • 28.7 x 6 – 46.7 = 126 mg/dL A1C EAG 5% 6% 7% 8% 9% 10% 11% 12% 13% 14% 97 mg/dL (5.4 mmol/L) 126 mg/dL (7 mmol/L) 154 mg/dL (8.5 mmol/L) 183 mg/dL (10.1 mmol/L) 212 mg/dL (11.7 mmol/L) 240 mg/dL (13.3 mmol/L) 269 mg/dL (14.9 mmol/L) 298 mg/dL (16.5 mmol/L) 326 mg/dL (18.1 mmol/L) 355 mg/dL (19.7 mmol/L) Diabetes Care 2008;31:1473-8 Relationship Between A1C and Average Blood Glucose Over Past 2-3 Months >10 Seriously >240 mg/dL Elevated 8-10 7-8 6-7 <6 Elevated Slightly Elevated Good Goal 183-240 mg/dL 155-183 mg/dL 126-154 mg/dL Non-Diabetes <126 mg/dL (Formula: 28.7 X A1C -46.7 = eAG) ADAG Study. Diabetes Care 2008. Target A1C Values • TN is a 42 y/o female with Type 2 diabetes. She has a 6 y/o child and a 14 y/o child – both have Type 1 diabetes. Her father is 75 y/o and also has diabetes. TN would like to know her goal A1C. Target A1C Values • TN is a 42 y/o female with Type 2 diabetes. She has a 6 y/o child and a 14 y/o child – both have Type 1 diabetes. Her father is 75 y/o and also has diabetes. TN would like to know her goal A1C. • What is TN’s goal A1C (per ADA)? • < 6% • < 7% • < 8% Target A1C Values • What is TN’s goal BG (per ADA)? • Fasting/preprandial? • < 100 mg/dL • < 110 mg/dL • 70-130 mg/dL • Postprandial? • < 130 mg/dL • < 140 mg/dL • < 180 mg/dL Target A1C Values • TN is a 42 y/o female with Type 2 diabetes. She has a 6 y/o child and a 14 y/o child – both have Type 1 diabetes. Her father is 75 y/o and also has diabetes. • What is the goal A1C for her 6 y/o? • Her 14 y/o? • Her father? Glycemic Control (2010 ADA Guidelines) A1C Goal Adults Children 0-6 <7% < 8.5% (> 7.5%) Higher goals due to hypoglycemia vulnerability <8% Age 6-12 Adolescents/ young adults Elderly < 7.5% ? Treatment of Type 1 DM • • • • Insulin MNT Exercise Other? • Pramlintide Role of Insulin • Suppresses • • • • Hepatic glucose production Lipolysis Proteolyis Gluconeogenesis • Promotes • • Transport of glucose into adipocytes/myocytes Glycogen synthesis Insulin Secretion • In adults without DM, the pancreas secretes 2550 units of insulin/day • Basal insulin secretion 0.5-1 units/hour • Additional insulin is secreted when BG > 100 mg/dL • Insulin is secreted in response to CHO intake at approximately 1 U/10-15 gram of CHO • In humans without DM, BG:40-160 mg/dL • BG> 40 mg/dL needed for normal brain function The Basal/Bolus Insulin Concept • Basal Insulin (Background insulin) • Suppresses glucose production between meals and overnight • Nearly constant levels • Supplies 50% of daily needs • Bolus Insulin (Mealtime or Prandial) • Limits hyperglycemia after meals • Immediate rise and sharp peak at 1 hour • 10% to 20% of total daily insulin requirement at each meal 6- NORMAL PANCREAS Insulin Effect ‘Bolus’ Insulin (Meal Associated) Basal Insulin (~0.5-1.0 U/hr) Insulin is released in response to varying blood glucose levels and hypoglycemia does not occur 6-23 Human Insulin Type Onset Peak Rapid Lispro/Aspart/Glulisine (Humalog/Novolog/Apidra) 5-15 min Regular (Humulin/Novolin) 30-60 min NPH (Humulin/Novolin) 2-4 hr 4-6 hr Detemir (Levemir) 2 hr 6-8 hr Glargine (Lantus) 2-4 hr Flat Premix Rapid Humalog Mix 75/25 Humalog Mix 50/50 Novolog Mix 70/30 Premixed Regular Humulin 70/30 Novolin 70/30 5-15 min 30-60 min 30-60 min 1-2 hr 2-3 hr Duration 4-6 hr 6-8 hr 14-18 hr 12 hr (0.2 U/kg) 20 hr (0.4 U/kg) 20-24 hr Dual 7-12 hr 14-18 hr Dual 7-12 hr Dual 7-12 hr 14-18 hr 14-18 hr Islet Cell Dysfunction Glucagon (alpha cells) Pancreas Glucose output Liver Insulin Amylin (beta cells) Blood glucose Glucose uptake Muscle Adipose tissue 1. Del Prato S,Marchetti P. Horm Metab Res. 2004;36:775–781. 2. Porte D Jr, Kahn SE. Clin Invest Med. 1995;18:247–254. Adapted with permission from Kahn CR, Saltiel AR. Joslin’s Diabetes Mellitus. 14th ed. Lippincott Williams & Wilkins; 2005:145–168. What is Amylin? • A 37-AA peptide hormone that is co-stored with insulin and co-secreted with insulin from the pancreatic ß cell in response to nutrient stimuli • Secreted in a pulsatile manner similar to insulin • Absent in Type 1 DM • Deficient in Type 2 DM • Pramlintide (Symlin®) is a synthetic analog of amylin Pramlintide (Symlin®) - MOA • Complements insulin in PPG homeostasis • Suppresses postprandial glucagon secretion from pancreatic cells • Neuroendocrine hormone – binds to CNS receptors • Effects mediated through the vagus nerve • Vagus nerve stimulates the gut • Slows gastric emptying • May enhance satiety through CNS activity • High-affinity binding sites in the area postrema in the hindbrain Pramlintide Side Effects • Nausea, fullness • Abates with continued use • ~ 4 weeks • Hypoglycemia • prandial insulin dose by 50% • Headache Drug interactions • Drugs that alter GI motility • Anticholinergics • Drugs that alter nutrient intake • AGIs • May delay absorption of concomitant meds • Give analgesics/OCPs 1 hr before/2 hrs after Type 1 DM Type 2 DM Pramlintide – Effects on A1C, BG, Weight, Insulin Dose • Overall, A1C 0.5 to 1% • BUT…PPG to near normal levels • 140-180 mg/dL • Possibly due to restoration of first-phase insulin secretion • Weight • 1 to 1.5 kg • Allows in insulin dose • Variable effect for each person SY • SY is a 28 y/o patient with Type 1 DM and her insulin regimen consists of Lantus 18 Units at bedtime and Humalog 6 Units with meals. Her A1C is 7.8% and she carb counts but she still has high PPG values. She especially loves to have cinnamon rolls on Tuesdays and Thursdays and then again on the weekends. • • • Is SY a candidate for pramlintide? What is the starting dose? If SY had Type 2 DM, what would be the starting dose of pramlintide? Trends in Type 2 Diabetes: 1988 - 2000 NHANES • Average BMI from 30.4 to 32.3 kg/m2 Pathophysiology of Type 2 DM • Two main factors • Insulin resistance • Hepatic, skeletal muscle, adipose tissues • Evident years before diagnosis • Impaired insulin secretion • Normal/ fasting plasma insulin • At diagnosis, ~ 40% of beta cell mass is left (due to apoptosis) Pathophysiology of Type 2 DM • Another main factor • Patients have • HTN • Hyperlipidemia • HIGH TGs • Low HDL • PAI-1 RE • RE is a 45 y/o male seen in clinic for balanitis and onychomycosis. The patient is 5’10” and weighs 240 lb. He complains of thirst and polyuria. His fasting glucose values have been in the low 120s (mg/dL). He has gained 40 lbs in the last 2 years. • Today, random glucose is 359 mg/dL • BP 148/98 mm Hg • Fasting lipids total cholesterol 240 mg/dL, triglycerides 438 mg/dL, HDL of 32 mg/dL • Does RE have Type 1 or Type 2 DM? Pharmacology & Pathophysiology GLP-1 Analogs Glucose Influx Sulfonylureas Glinides Insulin Alpha-glucosidase inhibitors Pramlintide Hepatic Insulin Glucose Hyperglycemia Secretion Output Biguanides (TZD) Insulin TZD (Biguanides) Insulin Peripheral Glucose Uptake Medications for Type 2 Diabetes • Biguanides – e.g., Metformin • Sulfonylureas • Thiazolidinediones (Glitazones) • Exenatide • DPP-IV Inhibitors • Glinides (Meglitinides) • Alpha glucosidase inhibitors • Colesevelam • Insulin AND… • Bromocriptine (Cycloset®) 2009 ADA/EASD Consensus Algorithm Tier 1 (Well-validated therapies) Step 1 At Diagnosis: Lifestyle (LS) + Metformin Step 2 Lifestyle (LS) + Metformin + Basal Insulin Step 3 Lifestyle (LS) + Metformin + Intensive Insulin Diabetes Care 2009;32:193-203 Lifestyle (LS) + Metformin + Sulfonylurea ADA/EASD Tier 2 – Less Validated Step 1 At Diagnosis: Lifestyle (LS) + Metformin Lifestyle (LS) + Metformin + Pioglitazone No hypoglycemia; Edema/HF; Bone Lifestyle (LS) + Metformin + GLP-1 agonist No hypoglycemia; Wt ; N/V Step 2 Lifestyle (LS) + Metformin + Pioglitazone + Sulfonylurea Diabetes Care 2009;32:193-203 Step 3 Lifestyle (LS) + Metformin + Basal Insulin Lifestyle (LS) + Metformin + Intensive Insulin RE • RE is a 45 y/o male seen in clinic for balanitis and onychomycosis. The patient is 5’10” and weighs 240 lb. He complains of thirst and polyuria. His fasting glucose values have been in the low 120s (mg/dL). He has gained 40 lbs in the last 2 years. • Today, random glucose is 359 mg/dL • BP 148/98 mm Hg • Fasting lipids total cholesterol 240 mg/dL, triglycerides 438 mg/dL, HDL of 32 mg/dL • What medication should be started? Biguanides - Metformin (Glucophage®) • MOA: hepatic gluconeogenesis • Other effects • Advantages • • • • Possible weight loss Rapid effects CVD benefits No hypoglycemia • Limitations • • • • GI side effects (titrate slowly) Renal dysfunction (Lactic acidosis risk) HF (but may use if HF is stable and Cr is normal) Females of childbearing age – RPh must counsel • Effects • A1C – 1-2% UKPDS, UKPDS 10-yr Follow-Up (RRR) UKPDS1 10-yr2 36% 27% 0 UKPDS1 10-yr2 39% 33% UKPDS1 10-yr2 30% 30% Meta analysis3 26% -5 -10 -15 -20 -25 -30 -35 -40 Mortality (all cause) P=0.01;0.002 1 Lancet 1998;352:854-65 2 N Engl J Med 2008;359:1577-89 3 Arch Intern Med 2008;168:2070-80 MI p=0.01;0.005 Macrovascular DM-related (all endpoints) death p=0.02 p=0.01 C-V Mortality 95% CI 0.62-0.89 Sulfonylureas • • • • Glipizide, glimepiride, glyburide MOA: Stimulate insulin secretion Monotherapy or combination Advantages • Rapid effects • Limitations • Weight gain • Hypoglycemia (Don’t delay/skip meals) • Elderly/ renal function • Benefit at half of max doses • 5-15% yearly secondary failure • Effects • A1C – 1-2% UKPDS 10-yr Follow-Up (RRR) 0 9% 17% 13% 15% 24% -5 -10 -15 -20 -25 DM related endpoints p=0.04 N Engl J Med 2008;359:1577-89 DM related Mortality death (all cause) p=0.01 p=0.007 MI p=0.01 Microvascular disease p=0.001 Thiazolidinediones • Pioglitazone (Actos®), Rosiglitazone (Avandia®) • MOA: Bind PPAR ( insulin sensitivity in muscle, fat, liver) • Monotherapy or combination • Advantages • Improves lipids • visceral fat/PAI-1 • Limitations • • • • Fluid retention/weight gain HF/cardiac events fracture risk Effect takes several weeks • Effects • A1C – 0.5-1.4% Alpha Glucosidase Inhibitors • Acarbose (Precose®), Miglitol (Glyset®) • MOA: Inhibit intestinal brush border enzymes that break down saccharides (e.g., CHO absorption) • Monotherapy or combination • Advantages • Weight neutral • PPG • Limitations • TID dosing • Slow titration • GI side effects • Effects • A1C – 0.5-0.8% Glinides • • • • Repaglinide (Prandin®), Nateglinide (Starlix®) MOA: Same as sulfonylurea (release insulin) Monotherapy or combination Advantages • Less hypoglycemia than sulfonylureas • PPG • May use in renal function • Limitations • TID dosing • weight • How to titrate repaglinide • Effects • A1C – 0.5-1.5% Bile Acid Sequestrant • • • • Colesevelam (Welchol®) MOA: Blocks glucose absorption Monotherapy or combination Advantages • Not absorbed; not metabolized • lipids • Limitations • Constipation, nausea, dyspepsia • TGs • May bind medications • Effects • A1C – 0.5% Dopamine Agonist • Bromocriptine mesylate (Cycloset®) • MOA: DA boost may re-set biological clock to improve metabolic problems • Monotherapy/Combination with SU, metformin/SU • Advantages • First “new” drug to follow FDA guidelines: Evaluated for potential CV adverse events (MI, stroke, other CV events) • May help lower elevated PPG; may weight • Limitations • Nausea/vomiting, HA, fatigue, orthostasis; lactation • “Psychosis;” May effectiveness of DA antagonists • May ergot side effects; CYP3A4 substrate • Effects • A1C ~ 0.5% HC • • • • • • • HC is a 56 y/o male with T2DM x 6 years On glyburide/metformin 5/500 mg – 2 po BID H/O CAD, HTN, IBS, NASH (fatty liver) FBG: 180-220 mg/dL PPG: 250-320 mg/dL A1C = 9.6% Should we? • • • • Intensify lifestyle? Add TZD? Add exenatide? Start insulin? Progressive Decline of -Cell Function-UKPDS 100 -Cell Function (% ) 80 60 40 20 0 -10 -9 -8 -7 -6 -5 -4 -3 -2 -1 Years 0 1 2 3 4 5 6 Adapted from UK Prospective Diabetes Study (UKPDS) Group. Diabetes. 1995; 44:1249-1258. Adding Insulin • If patient on 2-4 oral agents and A1C still elevated, time to add insulin • Typical delay when 2 oral meds fail? Adding Insulin • Add glargine 10 Units hs? • Advantage – can titrate every few days or weekly; less weight gain/hypoglycemia but expensive • Add NPH 10 Units hs? • Advantage – cost but must have good technique and patient may have hypoglycemia • Add levemir 10 Units hs or 5 units bid? • Advantage – can titrate; less weight gain/hypoglycemia • BUT…half of all patients eventually need prandial insulin Bottom line: Must talk to patient and individualize treatment Adding Insulin • When to add prandial insulin in Type 2 DM? • One opinion: • When basal insulin dose is > 40 to 50 Units and A1C > 7% • When basal dose approaches 1 Unit/kg and A1C > 7% • Add lispro 75/25 or aspart 70/30 twice/day? • Advantage – starting bolus and basal insulin; but more weight gain/hypoglycemia Bottom line: Must talk to patient and individualize treatment Islet Cell Dysfunction & Insulin Resistance Glucagon (alpha cells) Pancreas Glucose output Liver Gut GLP-1 Insulin Amylin (beta cells) Blood glucose Glucose uptake Muscle Adipose tissue 1. Del Prato S,Marchetti P. Horm Metab Res. 2004;36:775–781. 2. Porte D Jr, Kahn SE. Clin Invest Med. 1995;18:247–254. Adapted with permission from Kahn CR, Saltiel AR. Joslin’s Diabetes Mellitus. 14th ed. Lippincott Williams & Wilkins; 2005:145–168. The Incretin Effect GLP-1 restores first phase insulin secretion What Are The Incretins? • • • Gastrointestinal tract-derived hormones that are released in response to nutrient ingestion Approximately 60% of insulin secreted in response to a meal is due to the incretin effect 2 major incretins identified to date • Glucagon-like peptide 1 (GLP-1) • Released from L cells in ileum • Glucose-dependent insulinotropic peptide (GIP) • Released from K cells in jejunum What Are The Incretins? • Common actions of 2 major incretins • Exert effects on ß-cells to stimulate glucosedependent insulin secretion • Regulate ß-cell proliferation and cytoprotection • GLP-1 and GIP produce similar insulin release effects up to BG of 108 mg/dL • GIP has little effect at BG > 140 mg/dL Exenatide (Byetta®) • Synthetic GLP-1 analog • Synthetic version of exendin-4 (from Gila monster saliva) • Injectable • Side effects • • • • Nausea Hypoglycemia Pancreatitis Altered renal function Exenatide – Drug Interactions • May see hypoglycemia if given with SU • • Consider dose of SU before starting Dose reduction is a clinical judgment (~ ½) • May slow rate of absorption of concomitant orally-administered drugs • Take OCPs, antibiotics 1 hr before exenatide • If a concomitant med must be given with food, consider administering with a snack other than when exenatide is injected • Don’t use: Type 1 DM (or on insulin), ESRD, gastroparesis Exenatide (Byetta®) • Dose: Start out at 5 mcg bid (breakfast, supper) then increase to 10 mcg bid after at least one month if tolerated Exenatide (Byetta®) • Effects on A1C, BG, Weight • • • • A1C 0.5% to 1% FBG ~ 8 mg/dL PPG 60-70 mg/dL Weight is variable • In studies, up to 2.8 kg • May be greater in individual patients On The Horizon? • Exenatide Once Weekly • Advantage – once/week • 1.9% in A1C for 2 mg vs 1.7% for qd (10 mcg) • Disadvantage – unknown long-term side effects • Liraglutide (Victoza®) • Advantage - Once/day dosing • Disadvantage – a few patients developed small thyroid papillary carcinomas Glucagon Like Peptide 1 pathophysiology Mixed meal Long-term GLP-1 Actions: •Increase insulin synthesis •Promote ß-cell differentiation Intestinal GLP-1 release Active GLP-1 Acute GLP-1 Actions: •Augment glucose-induced insulin secretion •Inhibit glucagon secretion and hepatic glucose production •Slow gastric emptying •Increase glucose disposal Adapted from Rothenberg P. Diabetes. 2000;49(suppl 1):A39 Drucker DJ. Diabetes Care 2003;26:2929-2940. DPP-4 DPP-4 inhibitor Inactive GLP-1 DPP-IV Inhibitors • Sitagliptin (Januvia®) • Saxagliptin (Onglyza®) • Mechanism of action • • • Inhibit breakdown of GLP-1 and GIP Hence, levels of GLP-1 and GIP rise, especially in response to meals • This inhibits glucagon • Stimulates endogenous insulin secretion when glucose is highest Since these agents increase only glucosestimulated insulin secretion, there is little risk of hypoglycemia Gliptins • Side effects • • • • • Pancreatitis (within 30 days of start; metformin is protective) Headache Nasopharyngitis URIs (UTI with saxagliptin) Other concerns? • Thus far, no problems but theoretical concerns regarding the immune system since other DPP-IV substrates include growth factors and cytokines • DPP-IV may affect T-cell activity Gliptins • Sitagliptin dose adjustment in renal impairment • Cr Cl > 30 to < 50 mL/min is 50 mg daily • Males: Cr > 1.7 to < 3 mg/dL • Females: Cr > 1.5 to < 2.5 mg/dL • Cr Cl < 30 mL/min is 25 mg daily • Males: Cr > 3 mg/dL • Females: Cr > 2.5 mg/dL • On dialysis • Not studied in hepatic impairment • Saxagliptin 2.5 to 5 mg daily • 2.5 mg daily for CrCl < 50 mL/min DPP-IV Inhibitors • Gliptins: • If A1C is ~ 8-9% • A1C 0.5 to 0.8% • If A1C is 9-10% • A1C 1.4% • FBG ~16 to 22 mg/dL (sitagliptin); 10-15 mg/dL (saxagliptin) • PPG ~ 50-60 mg/dL (sitagliptin); 43-45 mg/dL (saxagliptin) • Weight neutral • Will help if close to A1C goals • Will help with decreasing PPG • Not evaluated in persons on insulin Monitoring? Drug Dose ADRs Cautions A1C Metformin 2000 mg/day GI; lactic acidosis Cr < 1.4 mg/dL Cr < 1.5 mg/dL 1-2% Sulfonylureas ½ of max dose; glyburide,glipizide (10 mg) glimepiride (4 mg) Hypoglycemia, weight gain, photosensitivity Do not skip or delay meals; weight, sunscreen 1-2% TZDs Pio – 45 mg/day Rosi – 8 mg/day Weight gain, fluid retention, HF, fractures LFTs, weight; baseline cardiac evaluation 0.5-1.4% Glinides Repaglinide – 16 mg/day Nateglinide – 120 mg/day Hypoglycemia, weight gain Weight, PPG 0.5-1.5% -glucosidase Inhibitors Acarbose/miglitol 50 mg tid GI; hypoglycemia Treat hypoglycemia with glucose 0.5-0.8% GLP-1 Agonists Exenatide 5 to 10 mcg bid GI; BG; pancreatitis Cut dose of SU by 1/2 0.5-1% DPP-IV Inhibitors Sita:50-100 mg/d Saxa:2.5-5 mg/d Nausea, infections pancreatitis Renal function; infections 0.5-0.8% Pramlintide 60 mcg/120 mcg Nausea, BG Dose of prandial insulin 0.5-1% Drug Class Insulin Biguanides % A1C Treat to target 1-2 Sulfonylureas TZDs Glinides Alpha-glucosidase Inhibitors 1-2 0.5-1.4 0.5-1.5 0.5-0.8 GLP-1 Agonists DPP-IV Inhibitors Pramlintide 0.5-1.0 0.5-0.8 0.5-1 Bile Acid Sequestrant Bromocriptine ~0.5 Monitoring Insulin • • • • • Blood glucose (fasting/postprandial) A1C Hypoglycemia Weight gain The dose that gets a person to target blood glucose/A1C (safely) is the right dose DM Control: How Intensive? Type 1 DM: Risk of Retinopathy JAMA 2002;287:2563-9 A1C Distribution After DCCT And Each Year During EDIC JAMA 2002;287:2563-9 Retinopathy:Cumulative Incidence EDIC Trial (JAMA 2002;287;2563-9) 7-Yr Incidence of Fatal/Nonfatal MI in Finland 7-Year incidence rate of MI (%) No diabetes (n=1,373) 50 45 40 35 30 25 20 15 10 5 0 Diabetes (n=1,059) 45 P<0.001 P<0.001 20 19 4 No previous MI* Previous MI No previous MI* *No previous myocardial infarction (MI) at baseline. Haffner SM et al. N Engl J Med. 1998;339:229-234. Previous MI Macrovascular Complications Treatment • Control of BG macrovascular complications in post active-intervention: • Type 1 DM: EDIC (at 17 years)1 • 42% in CVD outcomes (p=0.02) • 57% in risk of nonfatal MI, stroke, or CVD death (p=0.02) • Type 2 DM: UKPDS 10-year F/U2* SU + insulin: 15% RRR in MI (p=0.01) 17% RRR in DM-related death (p=0.01) 13% RRR in mortality (p=0.007) Metformin: 33% RRR in MI (p=0.005) 30% RRR in DM-related death (p=0.01) 27% RRR in mortality (p=0.002) *Criticized because of loss to F/U (selection bias?) 1 N Engl J Med 2005;353:2643-53 2 N Engl J Med 2008;359:1577-89 ACCORD ADVANCE VADT ACCORD • In T2DM pts with CVD or CVD risk, does intensive glucose control prevent CV events more than standard glucose control? • Goal A1C < 6% in intensive control group vs 77.9% in standard control • More CV mortality in intensive rather than standard control (trial stopped early) N Engl J Med 2008;358:2545-59 ACCORD • N=10,251 • Multiple drugs used to achieve goal (including 91% on rosiglitazone in intensive group) • Median BL A1C – 8.1% • Achieved A1C – 6.4% vs 7.5% N Engl J Med 2008;358:2545-59 ACCORD • Primary outcome • • • • Mortality • • • Nonfatal MI or stroke, CVD death HR 0.9 (95% CI 0.78-1.04) Significant? HR 1.22 (95% CI 1.01-1.46) (all-cause mortality) 257 vs 203 deaths Mortality higher if severe hypoglycemia, weight gain, on intensive insulin • Reason – Fast glucose lowering? N Engl J Med 2008;358:2545-59 ADVANCE • In T2DM pts does intensive glucose control prevent adverse events (microvascular + macrovascular) more than standard glucose control? • Goal A1C < 6.5% vs “based on local guidelines” • No difference in CV mortality between intensive and standard groups N Engl J Med 2008;358:2560-72 ADVANCE • N=11,140 • Compared gliclazide + multiple drugs (intensive) vs no gliclazide + multiple drugs (standard control) • < 20% received a TZD • Median BL A1C – 7.2% • Achieved A1C – 6.3% vs 7% N Engl J Med 2008;358:2560-72 ADVANCE • Primary outcome • • • • • Microvascular (nephropathy, retinopathy) + macrovascular disease (nonfatal MI or stroke, CVD death) Decreased mostly because of microvascular disease (specifically, nephropathy) HR 0.9 (95% CI 0.82-0.98) (microvascular disease) HR 0.94 (95% CI 0.84-1.06) (macrovascular disease) HR 0.93 (95% CI 0.83-1.06) (mortality) N Engl J Med 2008;358:2560-72 ADVANCE vs ACCORD • Comparison between the two studies • • • • • BL A1C lower than ACCORD (7.2% vs 8.1%) Duration of DM (2 yrs less) Less severe hypoglycemia in intensive gp (2.7% vs 16.2%) for ADVANCE BL BMI lower (28 vs 32) for ADVANCE Fewer on insulin in intensive gp (40% vs 77% at the end) for ADVANCE • ADVANCE verified risk with lower albuminuria if A1C to 6.3% VADT • In pts with long-standing T2DM (not wellcontrolled with insulin or max dose oral agents): • • Does intensive glucose control prevent CV events more than standard glucose control? Goal A1C < 6% (action if A1C > 6.5%) vs standard (target of 1.5% in intensive vs standard) • Results: • Intensive control had no effect on death, CV events, or microvascular complications N Engl J Med 2008;358:DOI:10.1056/NEJMoa0808431 VADT • N=1,791 • • • • Non-obese: rosiglitazone + glimepiride Obese: rosiglitazone + metformin Insulin if needed to reach goal 42% to 53% on TZD • Median BL A1C – 9.4% • Achieved A1C – 6.9% vs 8.5% N Engl J Med 2008;358:DOI:10.1056/NEJMoa0808431 VADT • Primary outcome • Nonfatal MI or stroke, CVD death, HF hospitalization, vascular disease surgery, inoperable CHD, ischemic gangrene amputation: • HR 0.88 (95% CI 0.74-1.05) • Mortality • HR 1.07 (95% CI 0.81-1.42) N Engl J Med 2008;358:DOI:10.1056/NEJMoa0808431 VADT vs ACCORD • Comparison between the two studies • Mortality increase NS in VADT • Endpoint A1C higher for VADT than ACCORD (6.9% vs 6.4%) • In VADT more hypoglycemia, weight gain, insulin use than in ACCORD • No difference in microvascular complications ACCORD F/U Information • Hypoglycemia was not a cause of death • Rate of glucose lowering not responsible for excess deaths • 3 BL factors emerged as predictors of increased mortality risk: • Higher BL A1C (> 8.5%) was associated with increased mortality • Reason? Possibly a surrogate for greater DM severity • H/O neuropathy • Reason? Surrogate for significant microvascular disease • ASA use • Reason? Surrogate for known/suspected CVD • Persons who got to goal did better in intensive group than those in standard group ADVANCE F/U Information • Risks/benefits of glucose lowering was uniform across different sub-groups • Intensive group had major reductions in microvascular disease without increased cardiovascular mortality • Those with greatest benefit attained optimal glucose and BP measures VADT F/U Information • Risk factors for primary CV event or total mortality: • • • • Hypoglycemia Previous CV event Older age Impaired renal function ACCORD, ADVANCE, VADT • Take home messages • • CVD Risk Management Critical • Manage BP, lipids, risk reduction (ASA, smoking cessation) Less stringent goals for glucose (A1C <7% not <6%) if: • H/O severe hypoglycemia • Limited life expectancy • Have micro or macrovascular complications • Long-standing DM where goals haven’t been achieved Macrovascular Complications Treatment • BP Management (per ADA) • Goal is < 130/80 mm Hg • Lifestyle (3 mo): • SBP 130-139 mm Hg • DBP is 80-89 mm Hg • Meds if BP > 140/90 mm Hg • ACE Is, ARBs, non dihydropyridine CCBs (if fail or can’t tolerate ACE Is or ARBs) Diabetes Care 2009 32(Suppl 1):S13-61 CARDS: Major CVD Events Cumulative hazard (%) 20 Placebo (n=1,410) Atorvastatin 10 mg/d (n=1,428) Primary prevention study in persons With DM and at least 1 risk factor 15 37% reduction P=0.001 10 5 0 0 1 2 Years CARDS=Collaborative Atorvastatin Diabetes Study 3 4 4.7 5 Lancet. 2004;364:685-696. Macrovascular Complications Treatment • Hyperlipidemia Treatment (per ADA) • If person doesn’t reach goal on max statin dose, lowering LDL by 30-40% from BL is alternative goal • May need concomitant meds to TGs or HDL • TGs do decrease if elevated A1C is decreased to goal Diabetes Care 2009 32(Suppl 1):S13-61 Macrovascular Complications Treatment • Hyperlipidemia Treatment (per ADA) • IF TGs are > 200 mg/dL: • Non HDL goal (TC – HDL) is 30 mg/dL higher than goal LDL • 2008 ACC/ADA guidelines: Measure Apo B • Represents most atherogenic lipoprotein particles • In children screen lipids at age 2 if FH positive or unknown; otherwise screen at puberty (> 10 years) Diabetes Care 2009 32(Suppl 1):S13-61 Macrovascular Complications Treatment • Risk Reduction • • • • ASA or other antiplatelets Smoking cessation (Immunizations) Lifestyle • Medical Nutrition Therapy • Physical activity Diabetes Care 2009 32(Suppl 1):S13-61 60 cardiovascular and microvascular events by 50% 50 P = 0.007 20 30 40 Conventional Therapy 10 Intensive Therapy 0 Primary Composite End Point (%) Multifactorial Intervention in Type 2 DM 0 12 24 36 48 60 Months of Follow-up N Engl J Med 2003;348:383-93. 72 84 96 HC • • • • • • • HC is a 56 y/o male with T2DM x 6 years On glyburide/metformin 5/500 mg – 2 po BID H/O CAD, HTN, IBS, NASH (fatty liver) FBG: 180-220 mg/dL;PPG: 250-320 mg/dL Wt 220 lb; BMI – 30kg/m2; A1C = 9.6% BP 142/85 mm Hg on lisinopril/HCTZ (40/25); HR - 90 Lipids: LDL only abnormal value (80 mg/dL) on Lipitor 20 mg • Should we? • • • • Intensify lifestyle? Add TZD? Add exenatide? Start insulin? HC - Plan • Should we? • Intensify lifestyle • Yes; send to a dietitian • Add TZD • No; h/o of CAD • Add exenatide? • Possibly; but pt has IBS and this is Tier 2 per ADA algorithm • Start insulin? • Yes; start with basal; titrate/treat to target (A1C: 3 mo) • Stop glyburide? • Prandial insulin when basal dose is ~ 50 Units/day (A1C: 3 mo) • Treat intensively to A1C < 6.5%? • No; goal is < 7%; monitor for hypoglycemia/weight gain • Intensify treatment of co-morbidities? • Yes; LDL goal is < 70 mg/dL; dose of Lipitor to 40 mg/day (recheck LDL and Apo-B in 4-6 weeks); monitor for ADRs • Yes; lifestyle for BP; add diltiazem 120 mg/day (re-check in 2 weeks); monitor BP and HR Role of the Clinician • Provide education • What is DM? • Target BG, A1C, BP, LDL • Information during pregnancy • Assess patient needs and provide MI • Provide information on how to recognize both hyperglycemia and hypoglycemia • Provide information on possible complications and how to avoid them (checklist) Role of the Clinician • Diabetes care checklist • • • • How to use a BG monitor; check pt’s technique Review BG log regularly, A1C goals Information on optimal medication use Remind pt of risk reduction (immunizations, smoking cessation, ASA use) • How to recognize hyperglycemia and a management plan • Sick day management instructions Management of Diabetes Goals A1C Plasma glucose (mg/dL) Preprandial Peak PPG BP Lipids TC LDL TG HDL Males Females < 7% 70-130 mg/dL < 180 mg/dL < 130/80 mm Hg <200 mg/dL <100 mg/dL (<70 mg/dL) <150 mg/dL >40 mg/dL >40 mg/dL >50 mg/dL Pre-Diabetes And Risk for DM? • • • • • • • My wt is > 20% of my IBW for ht (5 pts) I am < 65 y/o and do little/no exercise (5 pts) I am between 45-65 y/o (5 pts) I am > 65 y/o (9 pts) I am a woman who has had a baby weighing > 9 lb (1 pt) I have a sister/brother with DM (1 pt) I have a parent with DM (1 pt) Total # of pts scored: 3-9 pts: low risk, but note if in high-risk gp (wt, BP, ethnicity, etc.) > 10 pts: high risk; see HCP for further eval Pre-DM: Target values for BP and LDL same as DM