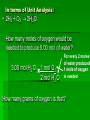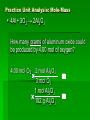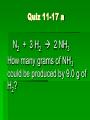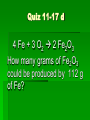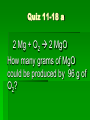* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File - Mrs. Roy`s Science Class
Artificial photosynthesis wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Isotope analysis wikipedia , lookup
Biochemistry wikipedia , lookup
Water splitting wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
History of molecular theory wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Electrolysis of water wikipedia , lookup
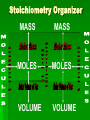
Stoichiometry Standard Students know how to determine the molar mass of a molecule from its chemical formula and a table of atomic masses and how to convert the mass of a molecular substance to moles, number of particles, or volume of gas at standard temperature and pressure. Students know how to calculate the masses of reactants and products in a chemical reaction from the mass of one of the reactants or products and the relevant atomic masses. Turkey Sandwiches 2 Slices of Bread + 2 Slice of Cheese + 5 Slices Turkey 1 Turkey Sandwich •If I have 6 slices of bread, how many sandwiches can I make? •How many slices of cheese and turkey will I need? •If I want to make 38 turkey sandwiches (one for each person in class), how many slices of bread, slices of cheese, and slices of turkey will I need? •How did you perform these calculations? Chocolate Chip Cookies This recipe yields 4 dozen cookies 1 cup butter, softened 1 cup white sugar 1 cup packed brown sugar 2 eggs 2 teaspoons vanilla extract 3 cups all-purpose flour 1 teaspoon baking soda 2 teaspoons hot water 1/2 teaspoon salt 2 cups semisweet chocolate chips 1 cup of chocolate chips = 60 g 1 cup flour= 125 g 1 cup butter= 227 g 1 cup sugar= 220 g If you have 908 g of butter, how many cookies can you make? How many grams of chocolate chips will you need? Stoichiometry Greek for “measuring elements” Study of quantities of materials consumed and produced in chemical reactions. Qualitative Quantative In terms of atoms and molecules: 2H2 + O2 2H2O Two molecules of hydrogen and one molecule of oxygen form two molecules of water. 2 Al2O3 Al + 3O2 2 molecules of aluminum oxide decompose to form 4 atoms of aluminum and 3 molecules of oxygen. In terms of Moles 2H2 + O2 2H2O Two moles of hydrogen and one mole of oxygen form two moles of water. 2 Al2O3 Al + 3O2 2 moles of aluminum oxide decompose to form 4 moles of aluminum and 3 moles of oxygen. In terms of Moles 2H2 2 moles 4 moles 10 moles 1 mole ? ? + O2 2H2O 1 mole ? ? ? ? 2.5 moles 2 moles ? ? ? 3.0 moles ? In terms of Unit Analysis: 2H2 + O2 2H2O How many moles of water could be produced by 2.00 mol of oxygen? 2.00 mol O2 2 mol H2O 1 mol O2 Ratio of coefficients How many grams of water is that? In terms of Unit Analysis: 2H2 + O2 2H2O How many moles of oxygen would be needed to produce 5.00 mol of water? 5.00 mol H2 O 1 mol O2 2 mol H2 O For every 2 moles of water produced, 1 mole of oxygen is needed How many grams of oxygen is that? Solving Stoichiometry Problems Write these steps on the left side of your notebook 1. Your “recipe” (chemical rxn) calls for moles. If you are given the amount in grams or molecules, convert to moles. 2. You are asked to determine how much ingredients you need (reactants) or how much of something you will end up with you will make (products). Use ratio of coefficients to find how much is needed. 3. If you are asked to find that amount in grams/molecules, convert moles to the unit you are asked to find. Mole to mass conversions (Molar mass) and mole to molecules conversions (Avogadro’s #) will always have the same molecule/atom in the numerator and denominator Ex1 mol Al2O3 102 g Al2O3 NOT 1 mol O2 102 g Al2O3 This is like saying… 1 dozen donuts 102 g eggs Practice Unit Analysis: Mole-Mass 4Al + 3O2 2Al2O3 How many grams of aluminum oxide could be produced by 4.00 mol of oxygen? 4.00 mol O2 2 mol Al2O3 3 mol O2 1 mol Al2O3 102 g Al2O3 Practice Unit Analysis: Mass-Mole How many moles of chlorine are required to react completely with 69.0 grams of sodium to produce sodium chloride? 2 Na + Cl2 2 NaCl 69.0 g Na 1 mol Na 23 g Na 1 mol Cl2 2 mol Na = 1.50 mol Cl2 Practice Unit Analysis: Mass-Mass Calculate how many grams of ammonia are produced when you react 28.0g of nitrogen with excess hydrogen. N2 + 3 H2 2 NH3 Stoichiometry Organizer MASS MASS MOLES MOLES VOLUME VOLUME Quiz 11-27 N2 + 3 H2 2 NH3 How many moles of H2 would be needed to produce 1.8 mol of NH3? Quiz 11-27a N2 + 3 H2 2 NH3 How many moles of H2 would be needed to react with 3.0 mol of N2? Quiz 11-28 a N2 + 3 H2 2 NH3 How many grams of NH3 could be produced by 1.0 mol of N2? Quiz 11-28 b N2 + 3 H2 2 NH3 How many grams of NH3 could be produced by 3.0 mol of H2? Quiz 11-28 c 2 Na + Cl2 2 NaCl How many moles of chlorine are required to react completely with 69.0 grams of sodium to produce sodium chloride? Quiz 11-28 d 4 Al + 3O2 2 Al2O3 How many grams of aluminum oxide could be produced by 27.0 g of aluminum? Quiz 11-16 N2 + 3 H2 2 NH3 How many grams of NH3 could be produced by 15.0 g of H2? Quiz 11-17 a N2 + 3 H2 2 NH3 How many grams of NH3 could be produced by 9.0 g of H2? Quiz 11-17 b 2 Na + Cl2 2 NaCl How many grams of NaCl could be produced by 140 g of Cl2? Quiz 11-17 c CH4 + 2 O2 CO2 + 2 H2O How many grams of H2O could be produced by 64 g of CH4? Quiz 11-17 d 4 Fe + 3 O2 2 Fe2O3 How many grams of Fe2O3 could be produced by 112 g of Fe? Quiz 11-18 a 2 Mg + O2 2 MgO How many grams of MgO could be produced by 96 g of O2? Quiz 11-18 b WO3 + 3 H2 W + 3 H2O How many grams of W could be produced by 6.0 g of H2? Quiz 11-29 N2 + 3 H2 2 NH3 How many grams of NH3 could be produced by 89.6 L of N2? Quiz 12-17b 2 Na + Cl2 2 NaCl How many grams of NaCl could be produced by 89.6 L of Cl2? Quiz 11-28 a 4 Al + 3O2 2 Al2O3 How many grams of aluminum oxide could be produced by 108 g of aluminum? Quiz 11-28 b 4 Al + 3O2 2 Al2O3 How many liters of oxygen would be needed to produce 34.0 g of aluminum oxide?