* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Matter_ properties_ mixtures and separation methods 2012
Survey
Document related concepts
Transcript
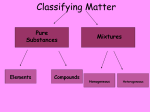
MATTER What it is all about Chemistry – Matter Unit • What is matter? • What is the organization of matter? • What is the nature of matter? MATTER Can the matter be separated by physical means? Mixtures Pure Substances Constant composition Heterogeneous Homogeneous Separation by chemical means Compounds Elements Matter Matter is anything that occupies space and has mass. The particle theory of matter. The particle theory states that… all matter is made from particles different particles have different properties particles are constantly in motion States of Matter A solid has a definite shape and volume. A liquid has a definite volume but no definite shape. A gas has neither a definite volume or shape. Gas Liquid Solid SOLIDS • the attraction between particles is strong so the matter holds its shape. The particles are still moving, but they are not able to slide past each other LIQUIDS • the attractive forces are not as strong. The particles are able to move past each other and slide around GAS • the attraction between particles is so weak that they fly in every direction filling the container that they are held solidification (freezing) melting Properties of Matter Physical Properties: • a quality or condition of a substance that can be observed or measured without changing the substance’s composition Examples: color, texture, boiling point, density, mass etc… Chemical properties • Properties that do change the chemical nature of matter • Properties the matter exhibits when chemical change occurs Ex. oxidation, flammability, corrosiveness, acidity Physical Properties Subcategories • Extensive Properties depend upon the amount of matter that is present. Ex. Length, mass, volume, heat …etc • Intensive Properties do not depend on the amount of matter present. These properties are the same for a given substance regardless of how much of the substance is present. Ex. Color, density, melting point, ductility, temp… etc Examples of physical properties • • • • • • • • • • • • • Boiling point Specific gravity (at constant temperature) Viscosity (at constant pressure and temp.) Freezing point Solubility in water (hot/cold) Density Melting point Mass Volume Specific heat capacity Heat Temperature Ductility/Malleability Density • the mass of a substance per a specific amount of volume Density = mass volume • The mass and volume are directly proportional. If one increases the other increases Physical and chemical change • Physical change – the altering of the physical form but not composition of matter – ex. Pounding, pulling, changes of state • Knowledge of physical change leads to… – the understanding of separation of mixtures – ex. Distillation, crystallization, chromatography, filtration Chemical change • Chemical change – change in which the matter is converted into matter with different composition and properties Indicators of chemical change 1. heat and/or light energy – Energy changes within the system 2. Production of gas - release of gas from the system 3. Formation of a precipitate - when two (or more) solutions are put together an insoluble solid is produced 4. Color change - the system changes color - not always an indicator of chemical change (can be physical MATTER Can the matter be separated by physical means? Mixtures Pure Substances Constant composition Heterogeneous Homogeneous Separation by chemical means Compounds Elements Pure Substances • Fixed composition • Cannot be separated into simpler substances by physical methods (physical changes) • Can only be changed in identity and properties by chemical methods • Properties do not vary Pure Substances Compounds • Can be decomposed into simpler substances by chemical changes, always in a definite ratio Elements • Cannot be decomposed into simpler substances by chemical changes MIXTURES mixture: - combination of two or more kinds of matter each of which retains its own composition and properties - physical blend of two or more substances More of Mixtures: heterogeneous mixture: - a mixture containing substances that are not evenly distributed - different from point to point ex. granite ---> quartz, feldspar, and mica Phase • mixtures that are obviously heterogeneous and have separate, distinct parts Ex. Oil forming layers in water is another Interface – the region where two or more phases meet MoM homogeneous mixture - a mixture containing substances that are uniformly distributed with the particles blended completely - composition and properties are uniform throughout - also called solutions (mixed on a scale of individual particles) ex. I molar copper II sulfate • To the eye, the mixture appears to be pure substance. Solutions (Homogeneous Mixtures) Can you tell the difference? Parts of a Solution • SOLUTE – the part of a solution that is being dissolved (usually the lesser amount) • SOLVENT – the part of a solution that dissolves the solute (usually the greater amount) • Solute + Solvent = Solution Solute Solvent Example solid solid Brass: Copper and Zinc solid liquid Seawater: NaCL in water gas solid Moth balls: naphthalene liquid liquid Ethyl alcohol and water (miscible) gas liquid SODA: CO2 gas in water gas gas AIR: O2 gas, N2 gas Some other terms to know! • Solubility – amount of substance that dissolves in a given quantity of solvent at specified conditions • Soluble – The ability to be dissolved • Miscible – Liquids that are able to dissolve in other liquids • Immiscible – Liquids that are insoluble in another liquid Somewhere In Between • Some mixtures are in-between heterogeneous mixtures and homogeneous solutions. • suspension • particles settle out upon standing • differ from a solution due to particle size being much greater (> 100 nm to 1 nm). • filtration to separate • colloid • smaller particle size than suspension but not as small as true solution • the particles are so small that they pass through most filters • fog is an example of a colloid • the Tyndall affect is exhibited https://www.youtube.com/watch?v=bn1-Zgcpp_4 Define the following words solubility. Miscible Immiscible Definitions Solutions can be classified as saturated or unsaturated. A saturated solution contains the maximum quantity of solute that dissolves at that temperature. An unsaturated solution contains less than the maximum amount of solute that can dissolve at a particular temperature Definitions SUPERSATURATED SOLUTIONS contain more solute than is possible to be dissolved in a given amount of solvent Supersaturated solutions are unstable. -- the supersaturation is only temporary -- need to warm the solvent so that it will dissolve more -- then need to cool the solution slowly http://www.youtube.com/watch?v=jVhPZg3dxIg http://www.youtube.com/watch?v=HnSg2cl09PI&feature=related Separation of Mixtures types of… • Filtration – Separation of mixture on the basis of differences in the size of the particles – Mostly used to separate solids from liquids (but filtration is used to separate all phases of matter from one another) Ex. Air filters separate gas (air) from solid (dirt particles) http://www.youtube.com/watch?v=nJVbFIIycKo https://www.youtube.com/watch?v=lfGYd1wrTgE • Distillation – Based on the tendency of a substance to vaporize (turn to a gas) – Based on boiling point differences – The substance in the mixture with the lowest boiling point will vaporize first from the mixture Ex. Crude oil http://www.youtube.com/watch?v=26AN1LfbUPc https://www.youtube.com/watch?v=JZdvsQzOKuk • Chromatography – Based on the differences in solubility – Two types Gas and Paper – Mixture separates as it travels (most soluble separates first) Solute – substance that gets dissolved Solvent – substance that does the dissolving http://www.youtube.com/watc h?v=LY44uD2miYM Ex. Separating ink in a marker http://www.youtube.com/watch?v=OKxRx0ctrl0&feature =related • Crystallization – Separation of the mixture is based on solubility differences – Temperature changes within the mixtures change solubility of parts of the mixture Solubility – the amount of a solute that is able to dissolve in a given amount of solvent Ex. Rock candy http://www.youtube.com/watch?v=l_USYub3djY&feature=related SOLUTION CHEMISTRY Concentration: • amount of solute in a given amount of solvent (can be determined quantitatively) Dilute: • a solution with a small amount of solute per solvent amount (relative term) Concentrated: • a solution with a large amount of solute per solvent amount (relative term) BOTH DILUTE AND CONCENTRATED ARE QUALITATIVE Concentration of Solute The amount of solute in a solution is given by its concentration. Molarity (M) = moles solute liters of solution The concentration of a solution is said to be its molarity. Ex. 1 M CuSO4 “1 molar copper II sulfate