* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Efavirenz-Central Nervous System Side Effects
Drug design wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Drug discovery wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Neuropharmacology wikipedia , lookup
Prescription costs wikipedia , lookup
Dydrogesterone wikipedia , lookup
Theralizumab wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Psychopharmacology wikipedia , lookup
Anti-Retroviral Therapy in South Africa
A pocket guide on the prevention and management of Side
Effects and Drug Interactions
DOH Emblem
FIRST EDITION
1
Anti-Retroviral Therapy in South Africa
A pocket guide on the prevention and management of Side
Effects and Drug Interactions
DOH Emblem
Writing Team:
Dr. Henry Fomundam
Medunsa Pharmacovigilance Centre
Dr. Christopher Mathews
The Eastern Cape HIV/AIDS Regional Training Centre &.HRSA Global AIDS Program, USA
Mr. Gustav N. Malangu
Medunsa National School of Public Health
Contributors :
Dr. MW Duma, Dr A. Grimwood, Dr. C. Khanyile, Dr. D. Kalombo, Dr. Z. Makatini, Dr. R. Mulumba , Dr.
V. Tihon
2
This pocket guide serves as a quick reference source for clinicians, in the management of patients on
antiretroviral drugs to complement treatment guidelines as outlined in the Comprehensive Plan for HIV
and AIDS Care, Management and Treatment. This booklet is a companion to other detailed guidelines
already available and it is to be used as a quick reference by trained healthcare workers. Information in
the pocket guide will be revised as necessary to reflect the dynamic nature of HIV and AIDS treatment.
Disclaimer: The Department of Health or the authors accept no responsibility for errors or omissions.
This pocket guide must be used in conjunction with the National Antiretroviral Treatment Guidelines and
other references.
3
TABLE OF CONTENTS
PREFACE .................................................................................................................................... 5
ACKNOWLEDGEMENTS............................................................................................................... 6
ACRONYMS AND ABBREVIATIONS.............................................................................................. 7
Section 1: ARVs regimens for drugs on the National Formulary ......................................................... 9
1.1. Adult Regimens .................................................................................................................. 9
1.2. Pediatric Regimens ........................................................................................................... 12
Section 2: Side Effects of ARV Drugs............................................................................................ 16
2.1. Efavirenz-Central Nervous System Side Effects................................................................... 16
2.2. AZT-Induced haematological Side Effects ........................................................................... 17
2.3. Dyslipidemia (Lipid Abnormalities) ...................................................................................... 20
2.4. Lipodystrophy ................................................................................................................... 22
2.5. Lactic Acidosis.................................................................................................................. 23
2.6. Gastrointestinal side effects ............................................................................................... 25
2.7. Allergies ........................................................................................................................... 29
2.8. Distal Symmetric Polyneuropathy (DSP) ............................................................................. 30
Section 3: Drug Interactions ......................................................................................................... 32
3.1. Drug-Drug Interactions ...................................................................................................... 32
3.2. Drug-Food Interactions ...................................................................................................... 46
Bibliography and Additional Information..................................................................................... 48
4
PREFACE
The first edition of “A pocket guide of the prevention and management of Side Effects and Drug
Interactions” in South Africa provides an easy and quick reference to assist the prescribers and those
responsible for clinical management of HIV and AIDS on the effective management of side effects and
drug interactions that are most common.
This is an evolving area, and as new information becomes available about drug interactions between
different medicines and antiretroviral drugs, as well as safety information from the pharmacovigilance
programme, further updates on a regular basis will be published. The Pharmacovigilance programme is
aimed specifically at collecting data from local settings where antiretroviral therapy will be used.
This text gives an outline of side effects, dosage regimen, and treatment for adverse drug reactions in
algorithms that are easy to follow. This reference must be read taking cognizance of the published
“National Antiretroviral Treatment Guidelines”.
Therapeutic regimens that have been selected for triple combination antiretroviral are limited to the
public sector comprehensive plan for the treatment, care and support of HIV and AIDS. Although not
exhaustive, more such publications will be available to support antiretroviral therapy and the safety
management of these therapeutic agents in the private sector.
The safety monitoring tools provided will serve as a sound basis to provide good safety standards. It is
envisaged that active reporting will be encouraged and a new culture created of reporting and sharing
experiences for better patient care and management.
Ms. M. P. Matsoso
Registrar of Medicines
Medicines Control Council (MCC)
5
ACKNOWLEDGEMENTS
The treatment of HIV, AIDS and opportunistic infections involves the use of several drugs. In South
Africa, a significant number of people use also alternative, complementary and traditional medicines.
The use of such a myriad of drugs calls for some guidance on rational drug use, as well as on
preventing and managing adverse effects, drug interactions and medications errors.
It is with pleasure that the National Department of Health wishes to acknowledge and thank all the
members of the writing team and contributors for developing such a much needed handbook.
Dr. R. Mulumba
Acting Chief Director and Cluster Manager: HIV, AIDS, and TB
6
ACRONYMS AND ABBREVIATIONS
3TC
Lamivudine
AIDS
Acquired Immune Deficiency Syndrome
ANC
Antenatal care
ART
Antiretroviral treatment
ARV
Antiretroviral
AZT
Zidovudine
D4T
Stavudine
ddI
Didanosine
EDL
Essential drugs list
EFV
Efavirenz
HAART
Highly active antiretroviral therapy
HBC
Home Based care
HIV
Human Immunodeficiency Virus
INH
Isoniazid
LPV
Lopinavir
M&E
Monitoring and evaluation
MCH
Maternal and child health
MTCT
Mother-to-child transmission
NNRTI
Non-nucleoside reverse transcriptase inhibitor
NRTI
Nucleoside reverse transcriptase inhibitor
NVP
Nevirapine
PEP
Post-exposure prophylaxis
PI
Protease inhibitors
PMTCT
Prevention of mother-to-child transmission
RTV
Ritonavir
TLC
Total lymphocyte count
VCT
Voluntary counselling and testing
7
8
Section 1: ARVs regimens for drugs on the National Formulary
1.1. Adult Regimens
Table 1: Adult regimens
Regimen
Drugs
1a
Lamivudine (3TC) + Stavudine (d4T) + Efavirenz
1b
Lamivudine (3TC) + Stavudine (d4T) + Nevirapine
2 (second Line)
Didanosine (ddI) + Zidovudine (ZDV) + Lopinavir/Ritonavir
For full dosing, consult the” National Antiretroviral Treatment Guidelines”
A. Antiretroviral naïve adult patients
Unless contraindicated, all patients will commence therapy on:
1. Stavudine (d4T) 40 mg every 12 hours (or 30 mg every 12 hours if < 60 kg), with
2. Lamivudine (3TC) 150 mg every 12 hours, and
3. Efavirenz (EFV) 600 mg at night (or 400 mg if < 40 kg) OR Nevirapine (NVP) 200 mg
first 2 weeks increasing to 200 mg every 12 hours after this.
daily for the
Note:
Ensure reliable contraception in women of childbearing age (preferably injectable contraceptive
and use of barrier method). If unable to guarantee reliable contraception, Nevirapine will be
substituted for Efavirenz. Extra safety bloods will need to be taken as per Table 2.
B. Antiretroviral non-naïve patients
Patients who have been previously exposed to antiretroviral therapy are to be discussed with a clinical
expert before a treatment regimen is commenced.
• Those patients controlled on their antiretroviral medication should continue on their treatment
or swap to the appropriate treatment protocol
• Those who stopped treatment for any reason but who were controlled, it is important to
establish the reasons for interruption, provide adherence counselling, and resume therapy
under close monitoring
• Those who have failed a previous regimen should be started on drugs they have not been
exposed to before and to which there is little likelihood of cross resistance as judged by a
clinical expert.
• Women and children who are eligible for antiretroviral therapy and whose only exposure to
antiretroviral drugs, previously was nevirapine used prevention of maternal to child
transmission (PMTCT) may have developed resistance to both nevirapine and efavirenz.
For these women, there is also a need to seek clinical guidance.
9
•
In general, clinical guidance could be obtained by contacting the HIV/AIDS Clinicians
Helpline: 0800 122 322
10
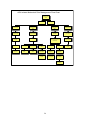
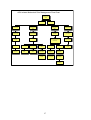
Figure 1:
Flowchart 1: First-line Treatment of Adults
(Regimen 1a – 1b)
Please note:
Patients who have been exposed to ARVs in the past need to be discussed with an
ARV expert BEFORE a treatment regimen is commenced.
All men & women on
injectable contraception +
condoms
Women who are unable
to guarantee reliable
contraception while on
therapy
1a
1b
1. stavudine (d4T) 40mg every 12
hours (or 30mg bd if <60kg) +
1. stavudine (d4T) 40mg every 12
hours (or 30mg bd if <60kg +
2. lamivudine (3TC) 150mg every
12 hours +
2. lamivudine (3TC) 150mg every
12 hours +
3. efavirenz (EFV) 600mg at night
(or 400mg if <40kg)
3. nevirapine (NVP) 200mg daily
for 2 weeks, followed by 200mg
every 12hours
Swapping drugs:
Swaps must be made by a doctor trained in anti retroviral therapy.
Figure 2:
Second-line antiretroviral therapy in adults (Regimen 2)
1. zidovudine (AZT) 300mg every 12 hours, with
2. didanosine (ddI) 400mg once a day (250mg daily
if <60kg), taken alone, dissolved in water on an
empty stomach, and
3. lopinavir/ritonavir (LPV/r) 400/100mg every 12
hours
11
Patients need to keep their
lopinavir/ritonavir safe, cool
&dry (<25°C)
1.2. Pediatric Regimens
Table 2: Pediatric regimens
First line
6months-3yrs old
Lamivudine (3TC) + Stavudine (d4T) + Lopinavir/Ritonavir
>3yrs old and > 10kg
Lamivudine (3TC) + Stavudine (d4T) + Efavirenz
Second Line
6months-3yrs old
Didanosine (ddI) + Zidovudine (ZDV) + Nevirapine
>3yrs old and > 10kg
Didanosine (ddI) + Zidovudine (ZDV)+ Lopinavir/Ritonavir
For full dosing, consult the” National Antiretroviral Treatment Guidelines”.
All infants under 6 months of age who require treatment with antiretroviral therapy should be
started on treatment under specialist supervision
Satvudine solution requires refrigeration. If no fridge available, stavudine capsules may be
opened and dissolved, and the required amount administered to the child. The rest can
be discarded
Kaletra is recommended for children under 3 years because it is assumed that most children in
this age group would have received nevirapine for PMTCT. Resistance mutations have
been shown to occur in a significant number of children exposed to nevirapine in this
fashion. Most resistance mutations have been shown to fade within the first year. It is
still unknown whether children with resistance mutation will have archived resistance
and therefore inadequate response to therapy with a regimen including an NNRTI.
Kaletra (Lopinavir/ritonavir) needs to be kept cool (< 25º C)
Didanosine must be taken alone, on an empty stomach, at least an hour before (or 2 hours
after) a meal. Tablets should be dissolved in at least 30 ml of water. It is important to
use 2 tablets of didanosine e.g if child needs 100mg prescribe 2x50mg tablets.
Drugs not listed in the 1st and 2nd line regimens such as ritonavir, nelfinavir, saquinavir,
abacavir, nevirapine may be available at tertiary care centres.
12
Table 3: Paediatric dosages per body surface area
Body surface (m2) Volume (ml) of Volume (ml) of Amount per dose
dose MORNING / 12hrs
each
dose each
MORNING / 12hrs MORNING / 12hrs later
later
later
0.30
0.35
0.40
0.45
0.50
0.55
0.60
0.65
0.70
0.75
0.80
0.85
0.90
0.95
1.00
1.05
1.10
Up to 1.4 BSA
ZIDOVUDINE
RITONAVIR
DIDANOSINE
10 mg/ml syrup
80 mg / ml syrup
25, 50, 100 mg
tablets
5.5 ml
6.0 ml
7.0 ml
8.0 ml
9.0 ml
10.0 ml
11.0 ml
12.0 ml
13.0 ml
13.5 ml
14.5 ml
15.0 ml
16.0 ml
17.0 ml
18.0 ml
19.0 ml
20.0 ml
1.5 ml
1.75 ml
2.0 ml
2.25 ml
2.5 ml
2.75 ml
3.0 ml
3.25 ml
3.5 ml
3.75 ml
4.0 ml
4.25 ml
4.5 ml
4.75 ml
5.0 ml
5.25 ml
5.5 ml
13
25 mg
25 mg
25 mg
25 mg
50 mg
50 mg
50 mg
50 mg
50 mg
75 mg
75 mg
75 mg
75 mg
75 mg
75 mg
100 mg
100 mg
CONTINUE
100
mg
EVERY 12 HRS UP TO 1.4
BSA
Table 4: Paediatric dosages per body weight
Weight Volume (ml) Volume (ml) Volume (ml) of EACH
EACH dose MORNING / 12
(kg)
of
EACH of
HRS LATER
dose
dose
MORNING / MORNING /
HRS
12
HRS 12
LATER
LATER
STAVUDINE LAMIVUDINE NEVIRAPINE
(d4T)
(3TC)
1 mg / ml 10 mg / ml 10 mg / ml
syrup
syrup
TWICE
TWICE
1-14 DAYS AFTER 14
ONCE
DAYS
TWICE
4
4 ml
1.5 ml
1.5 ml
3.0 ml
5
5 ml
2.0 ml
2.0 ml
3.5 ml
6
6 ml
2.5 ml
2.5 ml
4.0 ml
7
7 ml
3.0 ml
3.0 ml
5.0 ml
8
8 ml
3.0 ml
3.0 ml
5.5 ml
9
9 ml
3.5 ml
3.5 ml
6.0 ml
10
10 ml
4.0 ml
4.0 ml
7.0 ml
11
11 ml
4.5 ml
4.5 ml
8.0 ml
12
12 ml
5.0 ml
5.0 ml
8.5 ml
13
13 ml
5.0 ml
5.0 ml
9.0 ml
14
14 ml
5.5 ml
5.5 ml
10.0 ml
15
15 ml
6.0 ml
6.0 ml
10.5 ml
16
16 ml
6.5 ml
6.5 ml
11.0 ml
17
17 ml
7.0 ml
7.0 ml
12.0 ml
18
18 ml
7.0 ml
7.0 ml
12.5 ml
19
19 ml
7.5 ml
7.5 ml
13.5 ml
20
20 ml
8.0 ml
8.0 ml
14.0 ml
21
21 ml
8.5 ml
8.5 ml
15.0 ml
22
22 ml
9.0 ml
9.0 ml
15.5 ml
23
23 ml
9.0 ml
9.0 ml
16.0 ml
24
24 ml
9.5 ml
9.5 ml
17.0 ml
25
25 ml
10.0 ml
10.0 ml
17.5 ml
26
26 ml
10.5 ml
18.0 ml
27
27 ml
11.0 ml
19.0 ml
28
28 ml
11.0 ml
19.5 ml
29
29 ml
11.5 ml
20.0 ml
30
30 ml
12.0 ml
20.0 ml
31
30 ml
12.0 ml
20.0ml
32
30 ml
13.0 ml
20.0ml
33
30 ml
13.5 ml
20.0ml
34
30 ml
13.5 ml
20.0ml
35
30 ml
14.0 ml
20.0ml
36
30 ml
14.5 ml
20.0ml
37
30 ml
15.0 ml
20.0ml
14
Volume
(ml)
of
EACH dose
MORNING /
12
HRS
LATER
ABACAVIR
20 mg / ml
Amount
(mg) of
ONE DOSE
ONLY
(bedtime)
EFAVIRENZ
TWICE
50 and 200
mg caps
ONCE
1.6 ml
2 ml
2.4 ml
2.8 ml
3.2 ml
3.6 ml
4 ml
4.4 ml
4.8 ml
5.2 ml
5.6 ml
6 ml
6.4 ml
6.8 ml
7.2 ml
7.6 ml
8 ml
8.4 ml
8.8 ml
9.2 ml
9.6 ml
10 ml
10.4 ml
10.8 ml
11.2 ml
11.6 ml
12 ml
12.4 ml
12.8 ml
13.2 ml
13.6 ml
14 ml
14.4 ml
14.8 ml
200 mg
200 mg
200 mg
200 mg
200 mg
250 mg
250 mg
250 mg
250 mg
250 mg
300 mg
300 mg
300 mg
300 mg
300 mg
350 mg
350 mg
350 mg
350 mg
350 mg
350 mg
350 mg
350 mg
400 mg
400 mg
400 mg
400 mg
400 mg
NB: Please note that Abacavir doses should be rounded to the equivalent doses of Lamivudne.
15
Section 2: Side Effects of ARV Drugs
Prevention and management of side effects from drugs used to manage HIV and AIDS remain a
challenge to clinicians, patients, drug regulators, researchers, government, health care workers, family
members and all those affected. Acute and long term side effects, mild to severe (sometimes fatal)
reactions continue to affect patient decisions to start treatment, continue treatment, and adhere to
prescribed regimens. The clinician is also faced with the task of, selecting the right regimen, educating
or counseling the patient on possible side effects (prevention and management strategies) and
monitoring to ensure that benefits always outweigh the risk. A brief description and algorithms for the
management of common/severe adverse reactions with the regimens for the treatment of HIV on the
national formulary have been outlined for quick reference.
2.1. Efavirenz-Side Effects
Efavirenz is a potent NNRTI that acts by noncompetitive inhibition of HIV-1. CNS side effects have been
reported in more than 53% of people taking Efavirenz in some studies, with the most common ones
being dizziness, insomnia, impaired concentration, somnolence, abnormal dreams and hallucinations.
These side effects occur during the first 2 days of treatment and last for several hours after each dose.
Efavirenz neurologic symptoms are self limiting and generally resolve without treatment by the 4th
week, but can persist as mild symptoms for a longer time. These CNS effects can be aggravated by
psychoactive drugs or alcohol. Also manic and paranoid reactions as well as severe depression. Skin
rash has been reported up to 27% of patients but the most severe grade is limited to less than 5% which
could be Steven-Johnson syndrome.
According to Barlett and Gallant (2004), when initiating treatment with Efavirenz:
•
Prepare the patient: Screen and stabilize preexisting neuropsychiatric (NP) symptoms
•
Educate the patient: Regarding most common NP side effects
•
Reassure the patient: About Efavirenz’ effectiveness and the severity of its side effects which
are usually mild to moderate and of limited duration.
•
Treat: Address new and persistent side effects since early and effective management of CNS
side effects in the patient taking Efavirenz is imperative to improve patient outcomes.
Reminder: Efavirenz is contraindicated in women who are pregnant or breast-feeding; patients on
psycho-active drugs. Patients with history of psychiatric disturbances and seizures should be monitored
closely.
16
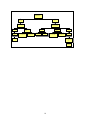
Management of Efavirenz-related CNS Side Effects
(adapted from:Canadian J of Infect Dis: July/August 2001, Volume 12, Number C)
1
Before starting treatment,
screen for pre-existing
substance abuse or psychiatric
symptoms including suicide
and depression
2
Yes
Pre-existing
symptoms present?
3
Evaluate and treat
symptoms
5
Educate patient regarding
risk and types of CNS side
effects.
6
4
If symptoms area moderate,
delay initiation of ARV or use
alternative agent instead of EFV
Dose initiation in
evening or at bedtime,
on empty stomach.
7
8
11
9 Evaluate potential causes
(psych, medical, substance
abuse).Minimize stimulants.
Suggest stress relieving
activities.\
10
Consider anxiolytics or
short term neuroleptic
(for severe case)
12
Adjust efavirenz
administration timing (eg,
give during the day).
13
Consider short term (2-3
weeks) benzodiazepine or
trazadone at bedtime.
Side Effects &
Management
14
Sleep
Disturbances.\
Agitation.\
No
17
15
20
Impaired
Concentration.\
Adjust efavirenz
administration timing
depending on when patient
experiences dizziness.
21
Adjust efavirenz administration
timing depending on when
impaired concentration occurs or
is most severe.
18
Adjust efavirenz
administration timing (eg,
give during the day).
16
Institute a trial of
short-acting
benzodiazepine
23
Dizziness.\
Disturbing Dreams.\
19
Assess home and
workplace safety for falls &
accident potential
22
Assess home and
workplace safety for
accident potential
Depression.\
24
Evaluate severity &
suicidality
25
Provide psychotherapy and
psychosocial support for
mild to moderate symptoms.
26Ensure patient
access to crisis
support services.
27 Provide close
patient follow-up or
monitoring.
28
Psychiatric referral &
antidepressent therapy
For severe depression or
suicidality, DISCONTINUE ARVs
or substitute EFV
2.2. AZT-Induced haematological Side Effects
Zidovudine, a NRTI was the first antiretroviral to be approved for the treatment of patients with HIV.
Common adverse reactions with AZT include, headache, malaise, myalgia, anorexia, nausea,
anemia and neutropenia. 5-10% of people taking AZT develop Anemia according to some studies.
Predisposing factors include, advanced stage of HIV infection, concurrent myelosuppressive agents or
chemotherapy. Anemia can be seen as early as 4 to 6 weeks after initiation of AZT. Hemoglobin levels
are usually used to evaluate the extent and progress of AZT-induced anemia.
Neutropenia occurs less frequently than anemia. Neutropenia usually occurs within 12 to 24 weeks of
initiating AZT. Neutrophil count can be used as a marker to determine the extent of AZT-induced
neutropenia. Predisposing factors also include, advanced stage of HIV infection and concomitant
myelosuppressive drugs. Granulocytopenia (very rarely thrombocytopenia) has also been reported with
AZT treatment.
17
AZT-related Hematologic Toxicity
AZT can cause anemia,
neutropenia, but not
thrombocytopenia
Hgb < 10 gm/dL
but > 8 gm/dL
Reduce AZT dose
to 200 mg twice
daily
Anemia on AZT
(usually
macrocytic)
Neutropenia on
AZT
Correct other causes
of anemia (e.g Iron
deficiency)
Calculate absolute
neutrophil count (ANC) =
WBC x %(segs+bands)
Hgb < 8 gm/dL or
25% decline from
baseline
Consult expert regarding
immediate replacement of
AZT with other NRTI
Hold all ARVs unless
making immediate
switch to other NRTI
ANC < 1500 but
>1000
Reduce AZT to
200 mg twice daily
ANC < 1000 or 50%
Decline from baseline
but no fever
Repeat FBC in 1
week
Consult expert regarding
immediate replacement of
AZT with other NRTI
Hold all ARVs unless
making immediate
switch to other NRTI
ANC < 1000 +
Fever
Hold ARVs
Obtain blood cultures &
Administer Ciprofloxacin
750 mg + Gentamicin at
once
Repeat Hgb in 1-2
weeks
Refer immediately
to hospital
18
19
2.3. Dyslipidemia (Lipid Abnormalities)
This is primarily reported with the Protease Inhibitors but have also been reported with the NRTIs and
NNRTIs. Increases in total cholesterol are usually due to PIs. NNRTIs are also known to increase total
cholesterol but have also been reported to increase HDL particularly Efavirenz. It is prudent to obtain a
fasting baseline serum lipid profile before initiating ART and take levels after 3 months. Other levels may
then be requested as clinically indicated depending on previous levels, cardiovascular risk factors or
symptoms. Life style modifications such as increased exercise, proper nutrition, weight loss, avoidance
of illicit drugs and alcohol and smoking cessation are all important measures to take to prevent or
decrease lipid abnormalities.
Dyslipidemia Management
(adapted from Dube et al. Clinical Infectious Diseases 2003; 37:613–27)
1
Obtain fasting glucose and lipid
profile prior to starting antiretrovirals
(protease inhibitors or efavirenz
containing) & w ithin 3-6 months of
starting new regimen\
2
Count number of CHD
risk factors &
determine level of risk\
3
Intervene for modifiable
non-lipid risk factors,
including diet & smoking.\
4
If above the lipid threshhold based on
risk group despite vigorous lifestyle
interventions, consider altering
antiretroviral therapy after
consultation w ith expert or use of lipid
low ering drugs.\
5
If lipid low er drugs
are necessary.\
6 Serum LDL cholesterol above
threshhold or triglycerides
2.26-5.65 mm/L (200-500 mg/dL)
w ith elevated non-HDL
cholesterol, STATIN therapy.
7
Serum triglycerides greater
than 5.65 mm/L (500
mg/dL), FIBRATE therapy.
20
21
2.4. Lipodystrophy
Fat redistribution has been reported with ART and typically involves, accumulation of visceral fat in the
abdomen (central obesity), dorsocervical area (buffalo hump) and breasts, loss of subcutaneous fat in
the face, extremities and buttocks.
Patients with fat redistribution should be screened for glucose (diabetes mellitus and glucose
intolerance) and lipid metabolism (high levels of triglycerides, total cholesterol, LDL cholesterol, low HDL
cholesterol) disorders. It is important that clinicians should monitor and recommend regular exercise,
proper nutrition and provide psychological support where necessary due to body habitus changes.
Various treatment strategies should be applied depending on the underlying cause.
22
Lipodystrophy Management
1
Key task is to distinguish
morphologic changes
associated w ith ARV therapy
from wasting due to HIV or OIs\
2
Phenotypes (suggest use
of provider & patient self
assessment scale,
anthropometrics)\
3
8
Wasting.\
4 Loss of fat and
muscle(lean body
mass).\
5
Nutritional.
6
Due to opportunistic
infection.
7
Due to uncontrolled
HIV infection (Wasting
Syndrome).
Fat loss
(lipoatrophy).\
14
Mixed fat loss &
gain.
9On NRTI containing
therapy (d4T most
associated)\
11
Fat gain
(lipohypertrophy).
15 common form,
Most
usually on NRTI + PI
containing therapy\
10
Consider
sw itch off
d4T to other NRTI
(consult expert).
12
On PI containing
therapy\
16Consider change
off d4T & change to
NNRTI based
13
Consider sw itch to
NNRTI based
regimen (consult
2.5. Lactic Acidosis
Lactic acidosis is a rare but life threatening condition and usually occurs in 1 to 20 months after start of
NRTI therapy. Clinical symptoms are non-specific and include, fatigue, nausea, vomiting, abdominal
pain, weight loss and dyspnea. These symptoms may occur acutely or gradually over time. A blood test
usually will show elevated levels of lactate with or without metabolic acidosis. A complete evaluation
should include an arterial blood gas, serum amylase and lipase levels and liver function tests.
Asymptomatic hyperlactatemia occurs more frequently, in about 15% of patients on NRTIs based on
some studies. Routine monitoring of serum lactate is not indicated nor recommended in patients with
asymptomatic hyperlactatemia. Levels should however be taken immediately if patient is symptomatic
23
and complains of fatigue, has sudden weight loss, abdominal disturbances, nausea, vomiting and
sudden dyspnea. Potential risk factors include female sex, obesity, prolonged exposure to NRTI
(especially D4T, DDI, or DDC), acute infection and pregnancy. Due to the fatality that has been reported
with lactic acidosis, such cases must be handled by or referred to experienced clinicians. The reason for
high mortality relates to the fact that the diagnosis is usually made late as clinicians treat these patients
for presumed P. pneumonia and think of lactic acidosis only when the patient fails to respond to
treatment. Therefore patients suffering from lactic acidosis should be referred to Hospital for inpatient
care since it persists for days even after the offending drugs have been discontinued.
Management of Lactic Acidemia
(Adapted from Carr, A., Clinical Infectious Diseases 2003; 36(Suppl 2):S96–100)
1
Consider spectrum of
symptomatic hyperlactemia lactic acidosis in patients on
NRTI therapy (especially d4T,
ddI, AZT)\
2
Symptoms may include: non-specific gastrointestinal symptoms with
or without mild ALT elevation, abdominal distention, nausea,
abdominal pain, vomiting, diarrhea, loss of appetite, shortness of
breath, ascending neuromuscular weakness, muscle aches, weight
loss, enlarged liver\
3
Measure serum electrolytes
& calculate anion gap (Na [Cl + CO2]); AG abnormal if
>16\
4
Measure venous lactate (drawn
without tourniquet, fluoride-oxalate
tube, on crushed ice and measured
within 4 hours)
Top of first nested tree\
5Lactate>10 mm/L
7Lactate 5-10 mm/L
with symptoms or
anion gap>16.\
9
with or without
symptoms.\
6
Discontinue ARVs and
refer immediately to
hospital.
8 Repeat lactate.
Stop ARVs & refer
to hospital
10
Lactate 5-10 mm/L
without symptoms or
elevated anion gap.\
Repeat lactate.
Dehydration or
laboratory artifact likely
13
11
Lactate 2-5 mm/L
with symptoms or
anion gap>16.\
12
Repeat lactate. If symptoms
worsenning & no alternative
explanation, stop ARVs and
refer to specialist
24
Lacate 2-5 mm/L
without symptoms or
elevated anion gap\
14
Monitor for development or
symptoms, continue therapy
15
Lactate < 2 mm/L\
16
Seek alternative explanation
of symptoms or elevated
anion gap.
2.6. Gastrointestinal side effects
Abdominal discomforts are the most commonly reported side effects with ARVs and may occur earlier
on in therapy. Common patient complains include, abdominal discomfort, nausea and vomiting, loss of
appetite, diarrhea, abdominal pain, pancreatitis, constipation and heartburn. Patients should be
informed that most gastrointestinal symptoms are self-limiting but some can linger for some time or
reappear and could be a sign of a serious condition. GI side effects can be a nuisance and greatly
impact drug therapy outcome and the patient’s quality of life. GI side effects can cause dehydration,
electrolyte imbalances, weight loss and malabsorption leading to low plasma drug levels. Coffee,
smoking, spicy food, unknown herbal medicines and non-steroidal anti-inflammatory products should be
avoided as much as possible. A workup should be done to diagnose the underlying cause or
complication of GI problems in order to take proper corrective measures. If diarrhea occurs, make sure it
is not of an infectious origin or lactose intolerance.
ARV-associated
Diarrhea Management
1
Yes
Diarrhea may be
associated w ith ddI &
protease inhibitors. Must distinguish
ARV-induced diarrhea from other causes
Is diarrhea associated w ith fever or
mucous/blood in the stool?
No
3
2
Not due to ARVs.
Evaluate and treat for
infectious diarrhea.
4
Yes
Possibly due to
ARVs.
Did
diarrhea begin
w ithin days-w eeks of
starting ARVs?
No
7
5
Administer antimotility agent
(if no fever, blood or
mucous in stool)\
Evaluate for other causes of diarrhea by
stool examination for WBCs, culture, and
parasite examination\
8
Treat based on test results
or syndromically for
infectious diarrhea
6If no improvement,
evaluate for other
causes of diarrhea.
25
ARV-related Abdominal Pain Management Flow Chart
Patient develops
abdominal pain on
ARVs
ARV related
Not ARV related
Pancreatitis (due
to ddI or d4T)
Hepatitis (due to
NVP, EFZ, AZT,
KTA)
Hyperlactatemia
(on d4T, ddI, or
AZT)
Ingestion related
Usually with
nausea, vomiting,
epigastric pain
May have yellow
eyes, light stool,
nausea, RUQ pain
Nausea, vomiting,
bloating, or
distention
Occurs within 1 h of
ingestion, crampy, &
goes away
Measure serum
amylase
Measure ALT
Measure venous lactate
(no tourniquet, place on ice
immediately)
Try taking with
food (unless ddI)
Amylase elevated
ALT 1.1 -2 XULN
ALT>2XULN
Jaundice
regardless of ALT
level
Lactate 2-5 mm/L
Lactate>5 mm/L
Refer to hospital &
hold ARVs
Repeat ALT in 1-2
weeks
Consult expert
about stopping
ARVs
Stop all
non-emergent
medications
Measure
electrolytes
Measure
electrolytes
Refer promptly to
hospital
Consult expert
Stop all ARVs
Refer to hospital
immediately
26
If no relief, consult
expert
ARV-related Abdominal Pain Management Flow Chart
Patient develops
abdominal pain on
ARVs
ARV related
Not ARV related
Pancreatitis (due
to ddI or d4T)
Hepatitis (due to
NVP, EFZ, AZT,
KTA)
Hyperlactatemia
(on d4T, ddI, or
AZT)
Ingestion related
Usually with
nausea, vomiting,
epigastric pain
May have yellow
eyes, light stool,
nausea, RUQ pain
Nausea, vomiting,
bloating, or
distention
Occurs within 1 h of
ingestion, crampy, &
goes away
Measure serum
amylase
Measure ALT
Measure venous lactate
(no tourniquet, place on ice
immediately)
Try taking with
food (unless ddI)
Amylase elevated
ALT 1.1 -2 XULN
ALT>2XULN
Jaundice
regardless of ALT
level
Lactate 2-5 mm/L
Lactate>5 mm/L
Refer to hospital &
hold ARVs
Repeat ALT in 1-2
weeks
Consult expert
about stopping
ARVs
Stop all
non-emergent
medications
Measure
electrolytes
Measure
electrolytes
Refer promptly to
hospital
Consult expert
Stop all ARVs
Refer to hospital
immediately
27
If no relief, consult
expert
28
2.7. Allergies
Allergies are a common occurrence with drug therapy. It however occurs more frequently in the HIV
population than in the Non-HIV patients. Rashes can occur with all ARVs but more common with
Nevirapine, Efavirenz and Abacavir. Allergy with Nevirapine and Efavirenz usually occurs within the
second or third week of treatment. It is usually an erythematous, maculopapular, pruritic, and confluent
rash distributed over the trunk and arm. Fever may precede the rash. Further symptoms include
myalgia, fatigue and mucosal ulceration. Severe but rare reactions such as Steven Johnson syndrome,
toxic epidermal necrolysis and hepatitis have been reported and will need prompt intervention by an
expert if it occurs.
Abacavir causes a hypersensitivity reaction (HSR) in 5-10% of patients which can be fatal. HSR is not
dose dependent and usually involves multiorgan systems. Abacavir HSR is characterized by fever and
usually accompanied by general malaise, nausea, vomiting, diarrhea and abdominal. Rash may occur
but is often mild. Abacavir must be discontinued and rechallenge is contraindicated. (Hoffman et al)
symptoms usually occur within 6 weeks, but can occur anytime.
ARV Rash Management Flow Chart
Patient is on Nevirapine
or Efavirenz containing
ARV regimen for less
than 1 month
Yes
Does
patient have any of
the following findings? Fever,
eye discomfort or blurred vistion, mouth or
genital sores, blisters on skin, rash is
hemorrhagic (doesn't
blanch)
No
STOP ALL ARVs and any
other medications
started within last
month
Stop any unnecessary
medications or traditional
remedies started within
last month
Refer immediately
to hospital
Teach patient
warning signs of
severe rash
Administer Benadryl
25-50 mg by mouth
every 6-8 hourly
Arrange daily follow-up
until rash either resolves or
becomes severe
29
2.8. Distal Symmetric Polyneuropathy (DSP)
It usually presents with a distal symmetric distribution and sensorimotor paralysis. Numbness or burning
dysesthesia of the distal extremities occurs at times with sharp shooting pains or continuous severe
burning. Signs of DSP include depressed ankle reflexes, abnormal vibratory pinprick and cold
sensations in the feet. Risk factors for DSP include, vitamin B12 deficiency, diabetes mellitus, history of
alcohol abuse, and neurotoxic drugs such as isoniazid (INH), history of DSP and advanced HIV/AIDS.
DSP is associated with several NRTIs with Zalcitabine > Didanosine > Stavudine > Zidovudine.
Don’t start ddI or d4T or ddC
containing regimen if patient has
symptoms of pre-existing neuropathy
If pre-existing neuropathic symptoms,
determine causes and treat (nutritional
and/or drug induced e.g. INH)
Neuropathic symptoms due to HIV
itself: diagnosis of exclusion
Peripheral
Neuropathy
Symptoms: Burning,
tingling pain of feet,
often worse at night.
Occasionally manifest
by leg cramps
Grade the severity of
symptoms and
relationship to start d4T
or ddI (Visual analog
scale 0-10; Gracely Pain
Scale)
Pain moderate of
severe
(not controlled with
NSAIDS +/adjuvants)
Pain Mild
(not requiring
opiates)
I.A.If no other NRTI substitution available (AZT), dose
reduce d4T by 10 mg bid
I. Discontinue all d-drugs (d4T, ddI,and/or ddC)
II. Substitute d-drug with other available NRTI (e.g. AZT)
after consultation with specialist
III. If no substitution NRTI feasible, must stop all ARVs at
once
IV. Treat pain with opiates and adjuvants (amitriptyline)
I.B.If AZT available as NRTI substitute, stop d4T or ddI or
ddC and replace with AZT
II. Treat pain with NSAIDs, acetominophen, and adjuvants
(amitriptyline, neurontin)
30
31
Section 3: Drug Interactions
3.1. Drug-Drug Interactions
Drug interactions have become an increasingly complex challenge for clinicians treating HIV-infected
patients.
Generally, drug interactions can be classified into two broad categories:
• interactions altering pharmacokinetics
• interactions affecting pharmacodynamics
Although both have the potential to be problematic in patients receiving HAART, pharmacokinetic
interactions are more common and more difficult to predict due to the complex nature of drug
metabolism. Most interactions are minor and may not be noticeable or of any clinical significance;
however there are equally a significant number of interactions that can cause a decrease in patient or
clinical outcomes, therapeutic failures, mild to moderate toxicity and severe to life threatening toxicities.
Clinically significant drug interactions are generally those that produce at least a 30% change in
pharmacokinetic parameters.
Drug interactions occur in almost all patients who are being treated for HIV/AIDS due to the average
number of drugs (for HIV and opportunistic infections), food interactions, vitamins, complementary and
herbal or traditional medicines that the patient may be taking.
A. Pharmacokinetic Interactions
Pharmacokinetic drug interactions can be classified according to whether they affect the absorption,
distribution, metabolism, or elimination of other drugs. Most common drug interactions encountered in
HIV infection involve those that affect metabolism or absorption.
Metabolism
Drug interactions involving metabolism are the most common and difficult to predict. Drugs used in
HAART, especially NNRTIs and PIs, are metabolized via the cytochrome P450 enzyme system
(CYP450). The CYP450 enzyme system is responsible for drug metabolism. The enzyme responsible
for the majority of drug metabolism is CYP3A4, although 2C19 and 2D6 are also common and, to a
lesser extent, CYP1A2. Drugs interact with CYP450 enzymes in one of three ways:
• through inhibition,
• through induction,
• by acting as a substrate
Some drugs may interact in more than one way and act as an inhibitor and inducer of different CYP450
enzymes. CYP450 enzymes are expressed both in the liver and in the enterocytes of the small intestine.
They could produce inhibition or induction of drug metabolism within the gastrointestinal tract. A
common example of this type of interaction is concurrent use of saquinavir and grapefruit juice. As a
result of CYP450 inhibition in the GI tract, grapefruit juice significantly increases the bioavailability of
saquinavir. Similarly, ritonavir may inhibit CYP3A4 in the intestine, which is one of the proposed
mechanisms that contributes to this drug acting as a pharmacokinetic “boost.”
Drugs that inhibit CYP450 enzymes generally lead to decreased metabolism of other drugs metabolized
by the same enzyme. The decreased metabolism can result in higher drug levels and increased
potential for toxicity. Although inhibition is usually reversible, irreversible inhibition of CYP450 can occur,
requiring new CYP450 enzyme to be synthesized to overcome the inhibition. Inhibition of drug
metabolism tends to occur quickly (based on drug half-life), with maximal effect occurring when highest
concentrations of the inhibitor are reached. Inhibition could be used therapeutically; for example ritonavir
is a very potent inhibitor of CY3A4, thus it used in combination with Lopinavir (Kaletra) to increase
Lopinavir blood levels. It is important to note that grapefruit juice contains various substances that inhibit
CYP3A4-mediated metabolism in the gut wall.
32
Induction of the CYP450 system results in the increased clearance of concomitant medications
metabolized by the same enzyme. When drugs that induce CYP450 enzymes are administered to a
patient, the body responds by increasing the production of specific enzymes of the CYP450 system. The
increased enzyme production could lead to increased metabolism and decreased concentrations of
drugs metabolized via the same pathway. In general, the maximal effect of enzyme induction is
apparent within 7 to 10 days, although with drugs with a relatively long half-life, such as methadone, the
full effect of induction may take even longer. Drugs may also undergo a phenomenon termed
“autoinduction”, whereby a drug has the capability of inducing its own metabolism. For example,
nevirapine is such a drug that is why it is dosed 200 mg daily for the first 14 days of treatment, then 200
mg twice daily thereafter. A drug may act as a substrate by occupying the active site of a specific
CYP450 enzyme. This drug’s metabolism is then affected by other medications that either induce or
inhibit the CYP450 enzyme system.
Absorption
Drug interactions that affect absorption occur when one drug reduces the bioavailability of a second
drug. Reduced absorption is caused by one of four mechanisms:
• alterations related to the presence or absence of food
• alterations in gastric pH caused by antacids, H2-blockers, or PPI
• chelation of drug caused by calcium, magnesium, or iron
• inhibition of the P-glycoprotein or other transport pump
B. Pharmacodynamic Interactions
Pharmacodynamic interactions occur when one drug causes an alteration in the pharmacological
response (drug effect) of a second without a resultant change in drug concentrations or pharmacokinetic
parameters. In this type of interaction, the pharmacological response from the drug can be antagonistic,
additive, or synergistic.
• Antagonistic effects result in the drug’s pharmacological effect being reduced due to concurrent
therapy, such as is seen when zidovudine and stavudine are co-administered.
• Additive effects occur when the use of two drugs leads to enhanced pharmacological activity
• Synergy occurs when the use of two or more drugs concurrently results in an effect that is
greater than the addition of all of the drugs together (i.e., the effect is exponential, not additive)
Table 5: Enzyme induction or inhibition of some ARV Drugs
Drug
Enzyme Substrate Will inhibit
Efavirenz
3A4, 2B6
3A4, 2C9/19
Nevirapine
3A4, 2B6
Lopinavir
3A4
3A4, 2D6
Ritonavir
3A, 2D6
3A, 2D6
33
Will induce
3A4
3A4, 2B6
1A2, 3A, 2C9
Table 6: Management of drug Interactions
ARV Drug
Interacting drug
Effect of interaction
Clinical
significance
Management
Abacavir
Alcohol
Decreased abacavir metabolism
by
alcohol
dehydrogenase.
Abacavir AUC: increased 41%;
half-life: increased 26%
●NCS
No dose adjustment necessary
Abacavir
Zidovudine
Abacavir
decreases
the
absorption
of
zidovudine.
Reduced Cmax
●NCS
No dose adjustment necessary
Abacavir
Lamivudine
Abacavir
decreases
the
absorption
of
lamivudine.
Reduced Cmax
●NCS
No dose adjustment necessary
Didanosine (ddi)
Tetracyclines
Magnesium and calcium ions
contained in the tablet’s buffer
chelate with the antibiotics
●PCS
Administer dll at least two hours
after or six hours before
tetracycline
Didanosine (ddi)
Atazanavir
The buffer in didanosine
neutralizes the acid environment
needed for Atazanavir absorption
●PCS
Didanosine buffered tablets
should be taken two hours after or
one hour before taking Atazanavir
to minimize the interaction or use
enteric coated tablets
Didanosine (ddi)
Tenofovir
The ddi AUC increases by 60%
●PCS
Patients taking drugs concurrently
require
dosage
reductions
according to their weight: >60kg:
250mg; <60kg:200mg;if severely
underweight give 125mg ddi once
daily
Didanosine (ddi)
Allopurinol
Inhibition
of
presystemic
metabolism by allopurinol. AUC of
ddi increased between 113%122%. Cmax increased 69-116%.
Increased ddi effects (pancreatitis
,neuropathy)
●PCS
Monitor patient for ddi side effects
Didanosine (ddi)
Ciprofloxacin
Chelation and adsorption of
ciprofloxacin by divalent and
trivalent ions contained in the ddi
buffer. AUC decreased 16%
Cmax decreased 28%.
●PCS
Administer ciprofloxacin at least 2
hours
after
didanosine
suspension
Didanosine (ddi)
Foods
Decreased didanosine effectsdue
to a reduction in bioavailability by
20-25% when given with any food
●PCS
Administer ddi at least 2 hours
apart with meals
34
Didanosine (ddi)
Itraconazole
Decreased
itraconazole
absorption due to decreased
gastric acidity resulting from
antacid buffer contained within
didanosine tablets/suspension.
Decreased itraconazole effects
●PCS
Administer itraconazole capsules
at least 2 hours after didanosine
tablets/suspension. Itraconazole
solution as suggested alternative
Didanosine (ddi)
Ranitidine
Inhibition of gastric acid slightly
enhancing
didanosine
bioavailablity by reducing acid
degradation. Ranitidine AUC:
decreased 16%; Cmax: no
significant change
●NCS
No dose adjustment necessary
Didanosine (ddi)
Metroclopramide
No significant
didanosine levels
to
●NCS
No dose adjustment necessary
Didanosine (ddi)
Loperamide
Didanosine AUC: no significant
change; Cmax: decreased 23%.
●NCS
No dose adjustment necessary
Didanosine (ddi)
Ketoconazole
Decreased
ketoconazole
absorption due to decreased
gastric acidity resulting from
antacid buffer contained within
didanosine tablets/suspension.
Possibly decreased didanosine
effects.
●PCS
Administer
didanosine
tablets/suspension at least 2
hours apart
Didanosine (ddi)
Ganciclovir
Decreased
oral
ganciclovir
absorption due to decreased
gastric acidity resulting from
antacid buffer contained within
didanosine tablets/suspension.
Didanosine AUC: increased
111%.
Ganciclovir
AUC:
decreased 21%
●PCS
Administer
didanosine
tablets/suspension at least 2
hours apart of oral ganciclovir
administration
Efavirenz
Midazolam and
triazolam
derivatives
In vitro studies suggest that
efavirenz is a potent inhibiter of
CYP3A4.There is a potential for
increased drug concentrations of
these medications and associated
toxicity.
●PCS
Caution required; monitor patients
for side effects of midazolam
Efavirenz
Clarithromycin
Concurrent use causes the
clarithromycin AUC and Cmax to
be decreased by 39% and 26%
respsectively
●CSI
Avoid concurrent use of the two
drugs. Clinicians may consider
using azithromycin instead, as
CYP450 drug interactions are
unlikely with this medication
Efavirenz
Methadone
Efavirenz is a CYP3A4 inducer
therefore leading to reduced
methadone levels as methadone
is metabolized by the same
isoenzyme. Effects are seen after
about one to two weeks or longer.
●PCS
When using the two drugs
concurrently monitor the patients
for signs and symptoms of
methadone withdrawal. Any
change in antiretroviral therapy
regimens should be reported to
the providers
35
change
Efavirenz
Rifampicin
Concurrent use of efavirenz with
rifampicin has been shown to
reduce the AUC and Cmax of
efavirenz by 26% and 20%
respsectively.
●PCS
Consult with experts before
considering the options below.
Increase efavirenz dosage to
800mgdaily. Substitute rifampicin
with rifabutin. Current guidelines
suggest that rifabutin dose be
increased to 450mg daily and the
efavirenz should remain 600mg
once daily.
Efavirenz
Phenytoin
Phenytoin induces the CYP450
system, blood drug levels of
efavirenz maybe reduced.
●PCS
Concurrent use of the two drugs
should be avoided if possible
Efavirenz
Phenobarbital
induces the CYP450 system
.Reduced drug levels of efavirenz
●PCS
Concurrent use of the two drugs
should be avoided if possible
Efavirenz
Indinavir
Both the indinavir and the
efavirenz affect the CYP3A4
system, blood levels of indinavir
maybe reduced
●PCS
Consult with experts before
considering the options below.
Increase indinavir dosage to
1000mg every eight hours
Efavirenz
Amprenavir
The amprenavir and the efavirenz
have an antagonistic effect on the
CYP3A4 system. Reduced doses
of the amprenavir occur.
●PCS
Standard dose for efavirenz.
Increase amprenavir dosage to
1,200mg three times daily
Efavirenz
lopinavir
The lopinavir and efavirenz have
an antagonistic effect on the
CYP3A4 system. The lopinavir
levels may be reduced
●PCS
Consult experts before
considering the options below:
Standard dose for efavirenz;
Increase lopinavir dose to
533mg/133mg (four capsules)
twice daily.
Efavirenz
Ritonavir
The ritonavir and efavirenz have
an antagonistic effect on the
CYP3A4 system. The ritonavir
levels may be reduced
●PCS
Consult experts before
considering the options below:
Standard dose for efavirenz.
Increase ritonavir dose to
533mg/133mg (four capsules)
twice daily
Efavirenz
Antacids
No significant effects
●NCS
No dosage adjustment is
necessary
Efavirenz
Carbamazepine
Not studied.Induction of CYP450
and CYP3A4 by both drugs may
lead to decreased efavirenz
●PCS
Avoid concurrent use. Consider
alternative
agents.
Monitor
carbamazepine levels and adjust
dosage accordingly
Efavirenz
Ergotamine
Inhibition of CYP450 3A4 by
efavirenz would result in
increased ergotamine effects
(e.g., ergotism).
●CSI
Do not co-administer
36
Efavirenz
Fluconazole
Inhibition of CYP450 3A4 by
fluconazole. Efavirenz AUC:
increased 16%; Cmax: no
significant change
●NCS
No dose adjustment necessary
Efavirenz
Itraconazole
Induction of CYP450 3A4 by
efavirenz results in decreased
itraconazole effects
●CSI
Do not co-administer
Efavirenz
Lorazepam
Lorazepam AUC: no significant
change; Cmax: increased 16%
●NCS
No dose adjustment necessary
Efavirenz
Phenobarbital
Induction of CYP450 3A4 by
Phenobarbital results in
decreased efavirenz effects
●PCS
Avoid combination if possible;
consider alternative agents;
monitor phenobarbital levels and
adjust dosage accordingly.
Suggested alternatives:
Gabapentin, Lamotrigine;
Topiramate
Efavirenz
St John’s wort
Induction of CYP450 3A4 by St.
John's wort results in decreased
efavirenz effects
●CSI
Do not co-administer
Efavirenz
Warfarin
Possible inhibition or induction of
CYP450 by efavirenz resulting in
increased or decreased warfarin
effects (altered INR, increased
risk of bleeding or clotting)
●PCS
Monitor INR or PT and adjust
warfarin’s dosage accordingly
Efavirenz
Phenytoin
Induction of CYP450 3A4 by both
drugs. Decreased efavirenz and
phenytoin effects.
●CSI
Avoid combination if possible;
consider alternative agents;
monitor phenytoin levels and
adjust as indicated. Suggested
drug substitutes are: Gabapentin
Lamotrigine, Topiramate
Efavirenz
Ketoconazole
Induction of CYP450 3A4 by
efavirenz.
Decreased
ketoconazole effects
●CSI
Avoid concomitant administration
Efavirenz
Azithromycin
Azithromycin AUC: no significant
change; Cmax: increased 22%.
●NCS
No dose adjustment necessary
Efavirenz
Clarithromycin
Inhibition of CYP450 3A4 by
efavirenz. Clarithromycin AUC:
decreased
39%;
Cmax:
decreased 26%; 14-hydroxy
clarithromycin AUC: increased
34%; Cmax: increased 49%
●PCS
Dose adjustment not established
Efavirenz
Ethinyl Oestradiol
Ethinyl estradiol AUC: increased
37%; Cmax: no significant
change.
●NCS
No dose adjustment necessary
37
Lamivudine
Cotrimoxazole
Lamivudine AUC: increased 44%.
Increased lamivudine effects
●NCS
No dose adjustment necessary
Nevirapine (NVP)
Methadone
NVP is a CYP3A4 inducer
therefore leading to reduced
methadone levels as methadone
is metabolized by the same
isoenzyme. Effects are seen after
about one to two weeks or longer.
●PCS
When using the two drugs
concurrently monitor the patients
for signs and symptoms of
methadone
withdrawal.
An
increase in methadone levels may
be necessary after the addition of
nevirapine
Nevirapine (NVP)
Oral contraceptives
Contraceptive failure may occur
due to induction of CYP3A4 by
NVP which increases metabolism
of the oral contraceptive.
●PCS
Use an alternative birth control
method
Nevirapine (NVP)
Rifampicin
Rifampicin and rifabutin are
potent CYP3A4 inducers which
reduce NVP trough levels by 37%
and 16% respectively
●PCS
In
patients
taking
antimycobacterial therapy substitute
rifampicin with rifabutin to
minimize reduction in neverapine
drug levels.
Nevirapine (NVP)
Phenytoin
Phenytoin induces the CYP450
system .Reduced drug levels of
NVP may occur.
●CSI
Concurrent use of the medication
should be avoided if possible
Nevirapine (NVP)
Carbamazepine
Carbamazepine induces the
CYP450 system .Reduced drug
levels of NVP may occur.
●CSI
Concurrent use of the medication
should be avoided if possible
Nevirapine (NVP)
Phenobarbital
induces the CYP450 system
.Reduced drug levels of NVP
●CSI
Concurrent use of the medication
should be avoided if possible
Nevirapine (NVP)
Indinavir
The indinavir and nevirapine have
an antagonistic effect on the
CYP3A4 system.
●PCS
Consult
experts
before
considering the options below:
Increase indinavir dosage to
1000mg every eight hours
Nevirapine (NVP)
Amprenavir
The amprenavir and NVP have an
antagonistic effect on affect the
CYP3A4 system. Amprenavir's
levels may be reduced
●PCS
Standard dose for NVP; Increase
amprenavir dosage to 1,200mg
TID
Nevirapine (NVP)
Atazanavir
Probable drug interactions may
occur although no data is
available
●PCS
Dosages have not yet been
established with NVP
Nevirapine (NVP)
Sir John’s wort
Induction of CYP450 3A4 by St.
John's Wort
●CSI
Avoid concurrent administration
Nevirapine (NVP)
Warfarin
Possibly decreased warfarin
effects (e.g., altered INR,
increased risk of clotting)
●PCS
Monitor INR or PT and adjust
warfarin’s dosage accordingly
38
Nevirapine (NVP)
Ketoconazole
Induction of CYP450 3A4 by
nevirapine. Ketoconazole AUC:
decreased
63%;
Cmax:
decreased 40%. Decreased
ketoconazole effects
●CSI
Avoid concurrent administration
Nevirapine (NVP)
Cimetidine
Inhibition of CYP450 3A4 by
cimetidine.
●NCS
No dose adjustment necessary
Nevirapine (NVP)
Clarithromycin
Nevirapine Cmin: no significant
change. Clarithromycin AUC:
decreased
29%;
Cmax:
decreased 20%; Cmin: decreased
46%; 14-hydroxy clarithromycin
AUC: increased 27%
●NCS
No dose adjustment necessary
Nevirapine (NVP)
Ethinyl estradiol
Induction of CYP450 3A4 by
nevirapine. Ethinyl estradiol: AUC
decreased
23%;
half-life:
decreased 44%; Norethindrone:
AUC decreased 18%; half-life:
decreased
15%.
Possible
contraceptive failure
●CSI
Avoid co-administration;
additional contraceptive
measures may be needed
Ritonavir/lopinavir
Efavirenz
Efavirenz is a potent inducer of
the CYP3A4 system. Significant
reductions in rotinavir levels may
occur when using these two drugs
concurrently. The AUC is reduced
by about 15%.
●PCS
Consult experts before
considering the following option.
Ritonavir/lopinavir dosage may
need to be increased
Ritonavir/lopinavir
Nevirapine
NVP is a potent inducer of the
CYP3A4 system. Significant
reductions in rotinavir levels may
occur when using these two drugs
concurrently. The AUC is reduced
by about 33%.
●PCS
Consult experts before
considering the following option.
Ritonavir/lopinavir dosage need to
be increased.
Ritonavir
Alprazolam
midazolam,
triazolam
Ritonavir inhibits the CYP3A4
system that metabolises the
benzodiazepams. Potential for
prolonged or increased sedation
or respiratory depression
●CSI
Avoid concurrent use. Substitute
with zolpidem ,oxazepam,
temazepam or lorazepam
Ritonavir
Simvastatin,
lovastatin, high
dose atorvastatin
Ritonavir inhibits the CYP3A4
enzymes responsible for the
extensive metabolism of the
statins. Statins’ levels are
markedly increased. Risk of
toxicity is increased i.e.
myopathy, renal failure and even
death
●PCS
Use pravastatin or fluvastatin
instead as they have minimal
effects on CYP450
,
39
Ritonavir
Rifampicin
Rifampicin is a potent inducer of
CYP3A4, leading to significant
reductions in PI levels potentially
leading to virologic failure or
resistance. May use with full dose
rotinavir
●PCS
Consider rifabutin
alternative
Ritonavir
Amiodarone
Increase in amiodarone levels
due to inhibition of CYP450 and
CYP3A4 by ritonavir. Increased
amiodarone effects
●PCS
Monitor amiodarone levels and
decrease its dosage accordingly
Ritonavir
Carbamazepine
Reduction in ritonavir and
increase in carbamazepine blood
levels
●PCS
Avoid concurrent use. Consider
alternative agents for
carbamazepine. Monitor
carbamazepine levels and adjust
dosage accordingly
Ritonavir
Cotrimoxazole
Induction of CYP450 3A4 by
ritonavir. Sulfamethoxazole AUC:
decreased 20%; trimethoprim
AUC: increased 20%
●NCS
No dose adjustment necessary
Ritonavir
Digoxin
Increased digoxin effects
●PCS
Monitor digoxin concentrations
closely and adjust dosage
accordingly
Ritonavir
Ergotamine
Increased ergotamine effects
●CSI
Do not use concurrently. Replace
with 5-HT agonists ("triptans")
Ritonavir
Fluconazole
Inhibition of CYP450 3A4 by
fluconazole. Ritonavir Inhibition of
CYP450 3A4 by fluconazole
●NCS
No dose adjustment necessary
Ritonavir
Itraconazole
Inhibition of CYP450 3A4 by
itraconazole resulting in increased
ritonavir effects
●PCS
Dose adjustment not established,
consult experts
Ritonavir
Metronidazole
Disulfiram-like reaction (e.g.
headache, hypotension, flushing,
vomiting) as a reaction with
alcohol in the Ritonavir Oral
solution
●CSI
Avoid concurrent use
Ritonavir
Phenobarbital
Induction of CYP450 3A4 by
Phenobarbital resulting in
decreased ritonavir effects
●PCS
Avoid combination if possible;
consider alternative agents;
monitor phenobarbital levels and
adjust accordingly. Possible
substitutes are Gabapentin,
Lamotrigine, Topiramate
Ritonavir
Sir John’s wort
Induction of CYP450 3A4 by St.
John's wort resulting in decreased
ritonavir effects
●CSI
Avoid concurrent use
40
as
an
Ritonavir
Warfarin
Possible inhibition of CYP450
3A4, 2C9 and 1A2 by ritonavir.
Resulting in decreased warfarin
effects (e.g., decreased INR,
increased risk of clotting)
●PCS
Monitor INR or PT and adjust
warfarin’s dosage accordingly
Ritonavir
Sildenafil
Inhibition of CYP450 3A4 by
ritonavir resulting in Sildenafil
increased blood levels and effects
such as hypotension, priapism.
●PCS
Consult with experts and initiate
therapy at 25 mg dose; do not
exceed 25 mg in 48 hour period.
Ritonavir
Phenytoin
Increased phenytoin blood levels
and effects.
●CSI
Avoid concurrent use; consider
alternative agents such as
Gabapentin Lamotrigine
Topiramate. Monitor phenytoin
levels and adjust its dosage
accordingly
Ritonavir
Nifedipine
Inhibition of CYP450 3A4 by
ritonavir. Increased nifedipine
effects (e.g., hypotension, cardiac
arrhythmias)
●PCS
Monitor and adjust nifedipine
dosage accordingly
Ritonavir
Fluoxetine
Inhibition of CYP450 2D6 by both
drugs. AUC: increased 19%;
Cmax: no significant change.
Increased
ritonavir
effects;
possibly increased fluoxetine
effects
●NCS
No dose adjustment necessary
Ritonavir
Theophylline
Possible induction of CYP450
1A2 by ritonavir. Theophylline
AUC: decreased 43%; Cmax:
decreased 32%; Cmin: decreased
57%; half-life: decreased 57%
●PCS
Monitor and adjust theophylline
as indicated
Ritonavir
Amitriptylline
Inhibition of CYP450 3A4 and
2D6 by ritonavir. Increased
amitriptyline effects (e.g., dry
mouth, hypotension, confusion).
Increased amitriptyline levels.
●PCS
Monitor and adjust amitriptyline
dosage accordingly
Ritonavir
Clarithromycin
Inhibition of CYP450 3A4 by
ritonavir. Clarithromycin AUC:
increased 77%; Cmax: increased
31%; Cmin: increased 182%.
Increased clarithromycin effects
●NCS
No dose adjustment necessary
41
Saquinavir inhibits the CYP3A4
system that metabolises the
benzodiazepams. There is a
potential for prolonged or
increased sedation or respiratory
depression. It’s the least potent
protease inhibitor on the CYP3A4
isoenzyme.
●CSI
Avoid concurrent use. Consider
substitution
with
zolpidem
,oxazepam,
temazepam
or
lorazepam
Simvastatin,
lovastatin,
high
dose atorvastatin
Saquinavir inhibits the CYP3A4
enzymes responsible for the
extensive metabolism of the
statins. Statins’ levels are
markedly increased. Risk of
toxicity is increased i.e.
myopathy, renal failure and even
death
●PCS
Select pravastatin or fluvastatin
as they have minimal effects on
CYP450 or use low dose of
artovastatin with close folllow-up
for potential hepatotoxicity
Saquinavir
Rifampicin
Rifampicin is a potent inducer of
CYP3A4 and CYP450, leading to
significant
reductions
in
saquinavir levels potentially
leading to virologic failure or
resistance. AUC: decreased 84%;
Cmax: decreased 79%
●CSI
Consider rifabutin as an
alternative . Avoid if possible; may
consider saquinavir 400 mg BID
with ritonavir 400 mg BID
Saquinavir
Garlic
Garlic induces the CYP3A4
enzymes resulting in significant
decrease in saquinavir levels.
Potential virologic failure or
resistance
●CSI
Avoid concurrent use particularly
when Saquinavir is the sole PI in
the regimen
Saquinavir
Carbamazepine
Induction of CYP450 and
CYP3A4 by carbamazepine may
reduce saquinavir levels
●PCS
Avoid concurrent use; consider
alternative
agents;
monitor
carbamazepine levels and adjust
dosage accordingly
Saquinavir
Fluconazole
Inhibition of CYP450 3A4 by
fluconazole. Saquinavir AUC:
increased 50%; Cmax: increased
56%.
●PCS
Dosage adjustments necessary
Saquinavir
Itraconazole
Inhibition of CYP450 3A4 by
itraconazole.
●NCS
No dose adjustment necessary
Saquinavir
Phenobarbital
Induction of CYP450 3A4 by
Phenobarbital. May decrease
saquinavir effects.
●CSI
Avoid combination if possible;
consider alternative agents;
monitor phenobarbital levels and
adjust dosage accordingly.
Suggested alternatives are:
Gabapentin, Lamotrigine
Saquinavir
Sir John’s wort
Possible induction of CYP450
3A4 by St John's Wort resulting in
decreased saquinavir effects.
●CSI
Avoid concurrent use
Saquinavir
Alprazolam
midazolam,
triazolam
Saquinavir
,
42
Saquinavir
Warfarin
Possible inhibition of CYP450 by
saquinavir. Increased warfarin
effects (e.g., increased INR and
risk of bleeding)
●PCS
Monitor INR or PT and adjust
warfarin as indicated
Saquinavir
Sildenafil
Inhibition of CYP450 3A4 by
saquinavir.
Sildenafil
AUC:
increased 200-1100%. Increased
sildenafil effects (e.g., headache,
flushing, priapism)
●PCS
Initiate sildenafil at 25 mg daily;
adjust dose as indicated; not
recommended to exceed 25 mg in
a 48-hours period
Saquinavir
Ranitidine
Inhibition of CYP450 3A4 by
ranitidine. Saquinavir AUC:
increased 67%; Cmax: increased
74%.
●NCS
No dose adjustment necessary
Saquinavir
Phenytoin
Induction of CYP450 3A4 by
phenytoin. May decrease
saquinavir levels
●CSI
Avoid combination if possible;
consider alternative agents;
monitor phenytoin levels and
adjust as indicated. Suggested
Alternative Agent(s): Gabapentin,
Lamotrigine,
Tiagabine,
Topiramate
Saquinavir
Ketoconazole
Inhibition of CYP450 3A4 by
ketoconazole. Increased
saquinavir effects
●PCS
Dose adjustment not established
Saquinavir
Grape fruit
Inhibition of gastrointestinal
CYP450 3A4 by grapefruit juice.
Saquinavir AUC: increased 50%;
increased saquinavir effects
●PCS
Separate grapefruit juice from
saquinavir administration by at
least 2 hours
Saquinavir
Clarithromycin
Inhibition of CYP450 3A4 by
clarithromycin. Clarithromycin
AUC: increased by 45%
●PCS
Dose adjustment not established
Saquinavir
Dexamethasone
Possible induction of CYP450
3A4 by dexamethasone. May
decrease saquinavir levels
●NCS
No dose adjustment necessary
Saquinavir
Erythromycin
Inhibition of CYP450 3A4 by
erythromycin. Increased
saquinavir effects
●PCS
Dose adjustment not established
Stavudine
Zidovudine
Competitive inhibition of
intracellular phosphorylation of
stavudine, with decreased
stavudine effects
●CSI
Avoid concurrent use
Stavudine
Didanosine
Cmax increased by 17%
●NCS
No dose adjustment necessary
43
Stavudine
Ethambutol
Concurrent use increases risk of
neuropathy
●CSI
Avoid concurrent use
Stavudine
Ethionamide
Concurrent use increases risk of
neuropathy
●CSI
Avoid concurrent use
Stavudine
Isoniazid
Concurrent use increases risk of
neuropathy
●CSI
Avoid concurrent use
Stavudine
Dapsone
Concurrent use increases risk of
neuropathy
●CSI
Avoid concurrent use
Stavudine
Zalcitabine
Concurrent use increases risk of
neuropathy
●CSI
Avoid concurrent use
Zidovudine
Stavudine
The thymidine analogues both
compete for the same
phosphorylation sites in the
growing chain of HIV DNA
●CSI
Avoid concurrent use
Zidovudine
Fluconazole
Zidovudine AUC: increased by
74%; Half-life: increased by
128%. Increased zidovudine
effects.
●NCS
No dose adjustment necessary
Zidovudine
Rifampicin
Avoid if possible; may consider
saquinavir 400 mg BID with
ritonavir 400 mg BID
●NCS
No dose adjustment necessary
Zidovudine
Valproic acid
Inhibition of glucuronidation of
Zidovudine, AUC: increased by
79%, and increased effects
●NCS
No dose adjustment necessary
Zidovudine
Phenytoin
Zidovudine clearance: decreased
by 30%
●NCS
No dose adjustment necessary
Zidovudine
Clarithromycin
Zidovudine Cmax: increased by
50%; AUC: no significant change
●NCS
No dose adjustment necessary
Zidovudine/
Lamivudine
Stavudine
The thymidine analogues both
compete for the same
phosphorylation sites in the
growing chain of HIV DNA
●CSI
Avoid concurrent use
Zidovudine/
Lamivudine
Valproic acid
Zidovudine AUC: increased by
100% with increased effects
●NCS
No dosage adjustment necessary
Zidovudine/
Lamivudine
Ganciclovir
Zidovudine AUC: increased by
19.5%; Cmax: increased by 62%
with increased effects
●PCS
Avoid combination if possible and
monitor and adjust dosage
accordingly
Zidovudine/
Lamivudine
Cotrimoxazole
Lamivudine AUC: increased by
44% and increased effects
●NCS
No dose adjustment necessary
44
Legend:
• Green=NCS => no clinically significant interaction, no action required
• Blue =PCS => potentially clinically significant interaction, require close monitoring, dose or and
timing adjustment as indicated
• Red =CSI => clinically significant interaction, these drugs should not be administered
concurrently or concomitantly.
45
3.2. Drug-Food Interactions
Food intake or meals can enhance or inhibit the absorption, metabolism, distribution and excretion of
drugs. Dietary management to improve the efficacy of a drug includes taking it with food, on an empty
stomach, taking it with particular foods or avoiding particular foods.
Table 7: ARV Drugs and Food Restrictions
Drug
Food Restriction
Efavirenz
Take on an empty stomach,
food seems to increase
absorption
Nevirapine
Not affected by food. Take
without regard to meals.
Stavudine
Give without regard to meals
Lamivudine
Take without regard to meals
(though
may
delay
absorption)
Take on an empty stomach,
Didanosine
1hr before a meal or 2hrs
after.
Zidovudine
Take with low fat meal
Lopinavir/ritonavir
Food significantly increases
plasma concentration. Take
with meals.
46
Other nutrient restrictions
Avoid alcohol
Buffered tablets can be
dispersed in clear apple juice
3.3. Herb/Traditional/Complementary-Drug Interactions
According to the National comprehensive treatment plan in South Africa, about 90% of HIV +ve patients
take some complementary or herbal medicine. This implies that a majority of patients on ARTs will also
be taking some form of herbal, traditional or complementary medicine. Research on herbal or traditional
medicines is very limited and thus have not been regulated for purity and potency. There is inadequate
clinician experience combining herbal, traditional or complementary medicines with ARVs. It is however
prudent that clinicians should document as much as possible the name, source and quantity of any
other medicines that the patient is taking. Clinicians should counsel patients on the possibility of drug
interactions that may result to therapeutic failure or toxicities.
The following complementary medicines have however been documented to have an effect on the
cytochrome p450 enzyme system:
•
•
•
•
•
•
•
St. John’s wort
Garlic
Ginseng
Melatonin
Milk thistle
Geniposide
Scullcap
47
Bibliography and Additional Information
For more information on Drug Interactions consult the following:
1. Websites:
• hivinsite.ucsf.edu
• www.hiv-druginteractions.org
• www.tthhivclinic.com
• www.aids-etc.org
• www.rx.com
• www.unaids.org
• www.medadvocates.org/marg/children/HIVTreatmentGuidelines/
•
2. Books
• South African Medicine Formulary. 6th Edition.
• National Antiretroviral Treatment Guidelines. 2004
3. Package inserts of registered ARV drugs
48