* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Anti-reflective coating wikipedia , lookup
Optical aberration wikipedia , lookup
Nitrogen-vacancy center wikipedia , lookup
Optical rogue waves wikipedia , lookup
Optical amplifier wikipedia , lookup
Rotational spectroscopy wikipedia , lookup
Nonimaging optics wikipedia , lookup
Atmospheric optics wikipedia , lookup
Fiber-optic communication wikipedia , lookup
Birefringence wikipedia , lookup
Photon scanning microscopy wikipedia , lookup
Ellipsometry wikipedia , lookup
Nonlinear optics wikipedia , lookup
Rotational–vibrational spectroscopy wikipedia , lookup
Retroreflector wikipedia , lookup
3D optical data storage wikipedia , lookup
Optical coherence tomography wikipedia , lookup
Silicon photonics wikipedia , lookup
Harold Hopkins (physicist) wikipedia , lookup
Passive optical network wikipedia , lookup
Chem 125 Lecture 29
11/12/08
This material is for the exclusive use
of Chem 125 students at Yale and
may not be copied or distributed further.
It is not readily understood without reference to
notes or the wiki from the lecture.
Pasteur's
"Bargain-Basement"
Moldy Racemic Acid
from Thann Pharmacy
(probably apocryphal anecdote via L. F. Fieser ~1960)
Purified l-(S,S)-Tartaric Acid ???
(Remember the smell of carvones!)
Penicillium glaucum had "eaten" (R,R)
React with One Enantiomer
Diastereomeric Reactions
Have Different Rates
React Racemate with
Resolved Chiral Reagent
or Catalyst (e.g. Enzyme)
Nature's Way
Prepare only one Enantiomer
Use resolved starting material
Or resolved reagent/catalyst
Eisai - 7389
Purchase 5 stereocenters. In starting materials
Make the rest.
Only One
1 by chiral Simulated Bed Chromatography.
Chiral Separation
2 by allyl silane additions.
2 by asymmetric dihydroxylations.
2 by oxy-michael reactions.
Specific or general selective
3 by asymmetric Ni/Cr reactions.
reactions that preferentially
1 by Jacobsen epoxidation.
form one isomer.
1 by conjugate reduction.
1 by enolate alkylation
1 by ketal formation
19
Stereocenters
Best
regards,
Frank
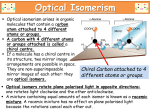
COOH
H
HO
OH
H
HO
H
H
OH
COOH
d-(+)
COOH
?
HO
H
H
OH
H
HO
OH
H
COOH
l-(-)
How does Optical Activity work?
Molecular Chirality and Optical Rotation
Laurence Barron
Chirality Means Right- or Left-Handedness
Chirality pervades much of modern science: from the
physics of elementary particles, through organic
stereochemistry, to the structure and behaviour of the
molecules of life, with much else besides (nonlinear
optics; nanotechnology; materials, electrical engineering;
pharmaceuticals; astrobiology; origin of life; etc.).
‘Rassi-maid’ by Hans Erni
Lord Kelvin (1824-1907)
Originally William Thomson. First introduced the word chirality into science.
Best known for inventing the absolute (Kelvin) temperature scale.
River Kelvin
Lord Kelvin’s Definition of Chirality
I call any geometrical figure or group of
points chiral, and say that it has chirality if
its image in a plane mirror, ideally
realized, cannot be brought into
coincidence with itself.
(Lord Kelvin, Baltimore Lectures, 1904)
Mirror-image chiral molecules
(enantiomers) show optical rotation of
equal magnitude but opposite sign.
(Louis Pasteur, 1848)
Natural Optical Rotation
Isotropic collections of chiral molecules (e.g. a sugar solution)
show natural optical activity phenomena such as optical
rotation.
Mirror-image enantiomers generate equal and opposite optical
rotation angles.
Not to be confused with magnetic optical rotation (the Faraday
effect).
Magnetic Optical Rotation
B
A static magnetic field B parallel to the incident light
beam induces magnetic optical rotation in
collections of achiral molecules (the Faraday effect,
1846). Reversing the magnetic field direction reverses
the sense of optical rotation.
Michael Faraday
(1791-1867)
Symmetry and Optical Rotation
“The magnetic rotation has neither right-handed
nor left-handed quality (that is to say, no
chirality). This was perfectly understood by
Faraday and made clear in his writings, yet even
to the present day we frequently find the chiral
rotation and the magnetic rotation of the plane of
polarised light classed together in a manner
against which Faraday’s original description of his
discovery contains ample warning.”
Lord Kelvin (Baltimore Lectures, 1904)
Chiral phenomena such as natural optical rotation are characterized by
time-even pseudoscalar observables. The quantum states of the
system must have mixed parity and definite reversality like Y(L) and Y(R).
Magnetic optical rotation is not a chiral phenomenon (the observable
is a time-odd axial vector). The quantum states must have definite parity
and mixed reversality like Y(J,M) and Y(J,-M).
Chirality and Circularly Polarized Light
In order to detect molecular chirality, some sort of chiral probe must be used.
Right- and left-circularly polarized light beams are mirror-image chiral
systems and so can act as chiral probes:
right
z
clockwise
Changing
Time
Fixed
atTime
Fixed
Position
The instantaneous electric field
vectors of right- and leftcircularly polarized light beams
propagating along z.
counterz clockwise
left
Chiral molecules respond slightly differently to right- and left-circularly
polarized light. A difference in absorption corresponds to circular dichroism; a
difference in refractive index leads to optical rotation.
Circular Differential Refraction
Linearly polarized light can be described as a
coherent superposition of right- and left-circularly
polarized waves of equal amplitude.
A difference in refractive index for the right- and
left-circularly polarized beams means a difference
in velocity. So the phase relation between the two
contrarotating electric vectors will change, resulting
in a rotation of the plane of polarization.
l L
(n n R )
Go to Google Images for animations (Google
‘circularly polarized light’ and open the
www.enzim.hu site).
From P.W. Atkins, Physical Chemistry (OUP)
A Scattering Picture of Optical Rotation
A circularly polarized light wave ‘bouncing’ from one group to the other
as it scatters from a simple two-group chiral molecular structure will
sample the chirality. The scattered intensity of right- and left-circularly
polarized waves will be slightly different for a given handedness of the
chiral structure.
The Rotational Strength
The optical rotation angle is given by
l L
(n n R )
Using quantum-mechanical perturbation theory this becomes
2mo lN
2
Im n m j
2
2
3
jn jn
j mn
2c/
The molecular quantity responsible for optical rotation (and circular
dichroism) is the rotational strength:
R j n Im n m j
j m n .
m and m are electric and magnetic dipole moment operators, respectively,
so the optical activity ultimately originates in interference between electric
and magnetic dipole transitions during the light scattering process!
The Carbonyl Chromophore
The carbonyl chromophore is an important
source of optical activity in many organic
compounds. The carbonyl group itself has a
plane of symmetry so is not chiral/optically
active. Optical activity is induced in its
electronic transitions via perturbations from
the chiral environment provided by the rest
of the molecule.
n
transition at ~ 290 nm
* n transition is magnetic dipole-allowed, electric dipole-forbidden.
Electric dipole character is induced by mixing of the oxygen dYZ orbital into
the orbital:
C
O
C
O
C
O
*
C
O
C
O
C
O
x
z
y
n
* n
n
dXZ n
linear displacement
of charge
(electric)
rotation of charge
(magnetic)
+ dXZ) n
helical motion
of charge
(both)
This generates a mZmZ component (from m.m) of the rotational strength:
R dYZ n Im n mZ dYZ dYZ mZ n
Quantum-Chemical Calculations
2mo lN
2
Im n m j
2
2
3
jn jn
j mn
The optical rotation can be calculated using ab initio quantum-chemical
programs (Gaussian, Dalton). Often sufficiently good to determine
absolute configuration from the sign and magnitude.
(S)-()-thalidomide
(teratogenic)
(R)-)-thalidomide
(sedative)
Tetelman Lecture
From Cosmic Chirality
to Life on Mars:
Lord Kelvin’s Legacy
Davies Auditorium
Becton Center
5:00 PM Today
Who Cares?
Living Things
Food & Drug Administration
Drug Companies
their Lawyers & USPTO (Patent Office)
"Chiral Switch"
Pain Reliever
Ibuprofen (Advil, Motrin)
COOH
Isobutyl
Phenyl
Propionic
Acid
(S) Active Pain Reliever
(R) Inactive
Sold as racemate
Sedative
H
O
N
Thalidomide
O
O
N
O
(S) Sedative
(R) Teratogen
Sold as racemate (1957-62)
10,000 birth defects
in vivo racemization (human)
(S) (R) 0.12 / hr
(R) (S) 0.17 / hr
?
(S) eliminated 0.24 / hr
(R) eliminated 0.08 / hr
5-Methoxy-2-{[(4-methoxy-3,5-dimethyl-2pyridinyl)methyl]sulfinyl}1H-benzimidazole
Gastric Proton Pump Inhibitor (Acid Reflux)
World's largest selling drug in 2000 ($6.2B)
"Omeprazole"
"Prilosec"
4
3
5
2
6
1
3
•• O
4
9
2
5
1
8
omeprazole
Benzimidazole
Pyridine
7
6
OTC?
5-Methoxy-2-{[(4-methoxy-3,5-dimethyl-2pyridinyl)methyl]sulfinyl}1H-benzimidazole
Gastric Proton Pump Inhibitor (Acid Reflux)
World's largest selling drug in 2000 ($6.2B)
"Omeprazole"
(racemate)
"Prilosec"
••••••O
"esomeprazole"
"Nexium"
(S)
O
B I N O C U L A R SS TT EE RR EE O
N
OO
NN
OO
O O
N
Stereoviewing
(S)-Omeprazole - Stereopair View
from X-rayof Ohishi et al. 1989
left eye
fromX-ray of Ohishi et al. 1989
right eye
(S)-Omeprazole - Stereopair View
from X-ray of
Ohishi et al. 1989
How it should
look when you
stare dreamily into
the distance
“through” the
stereo-pair above
until the blue lines
“swim” together
and superimpose.
right-eye view
left-eye view
Central frame
perceived in stereo
End of Lecture 29
Nov. 12, 2008