* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Atoms and Moles
Survey
Document related concepts
Transcript
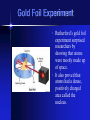
Atoms and Moles The Atomic Theory • As far back as 400 BC, people believed that the atomic theory existed. • The atomic theory basically stated that atoms are the building blocks of all matter. • This theory had no experimental results to back it up at the time. John Dalton • In 1808, an English schoolteacher named John Dalton used the Greek concept of the atom along with 3 other scientific laws to develop an atomic theory. • He believed that all matter was made up a few kinds of atoms. • He also stated that all elements were made up of only one type of atom and that compounds were made up of two or more types of atoms. Dalton’s Atomic Theory • All matter is composed of extremely small particles called atoms that cannot be subdivided, created, or destroyed.* • Atoms of a given element are identical in their physical and chemical properties. * • Atoms of different elements combine in simple, whole number ratios to form compounds. • Atoms of different elements differ in their physical and chemical properties. • In chemical reactions, atoms are combined, separated, or rearranged but never destroyed, created, or changed. The Laws Involved: • The Law of Conservation of Mass: The mass of reactants equals the mass of the products in a chemical reaction. Mass cannot be created nor destroyed. • The Law of Multiple Proportions: If two or more different compounds are composed of the same elements, the ratio of the masses of the second element is always the ratio of small whole numbers. • Law of definite proportions: Samples of a given compound are made with the same elements in exactly the same proportions by mass regardless of the size or the source of the samples. Electrons • Electrons were discovered in the mid-1800’s by J.J. Thompson. • The electron has a negative charge and is depicted as e,e-, 0-1 e. • It’s charge is -1.602x10 -19 C. • It’s mass is 9.109x10-31 kg. Cathode ray tube • The cathode ray tube was used by JJ Thompson to discover electrons. • Magnets or charged plates can be used to attract or deflect the beam which proved that the beam had a negative charge. Protons • Protons were discovered shortly after the electron. • The proton has a positive charge and is depicted as p,p+, or 1+1p. • It’s charge is +1.602x10-19 C. • The mass of a proton is 1.676x10-27 kg. Neutrons • Discovered by British scientist James Chadwick. • Neutrons carry no charge. They are electrically neutral. • The charge is 0 C. • They can be depicted as n or 01n. • The mass of a neutron is 1.675x10-27 kg. Gold Foil Experiment • Rutherford’s gold foil experiment surprised researchers by showing that atoms were mostly made up of space. • It also proved that atoms had a dense, positively charged area called the nucleus.