* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Periodic Law
Boron group wikipedia , lookup
Group 12 element wikipedia , lookup
Alkali metal wikipedia , lookup
Alkaline earth metal wikipedia , lookup
Group 3 element wikipedia , lookup
Period 3 element wikipedia , lookup
Period 6 element wikipedia , lookup
Period 2 element wikipedia , lookup
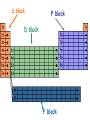
Periodic Law Chapter 6 Objectives 1. History of the Periodic table 2. Start talking about Periodic trends 3. Periodic Table Geography … families of elements 1790 Antoine Lavoisier – Compiled a list of known elements – 23 1864 John Newlands – 1st proposed organizational scheme for elements Dmitri Mendeleev – Russian Chemist who first developed the Periodic Table. Mendeleev’s Periodic Table – elements are arranged according to increasing atomic mass 1st Draft Version of Mendeleev’s table In 1913 Henry Moseley conducted X-ray experiments on elements. The outcome of his work was the introduction of the atomic number. It was found that if Mendeleev's table was ordered by atomic number instead of atomic mass the inconsistencies in the table were eliminated. This is the blueprint for the modern periodic table. Periodic Law – The physical and chemical properties of the elements are periodic functions of their atomic numbers. Periodic Trends Atomic Radii –The size of an atom – one half the distance between the nuclei of two identical atoms bonded together Atomic Radii • Decreases as you go across a period due to the added positive charge to the nucleus. • Increases down a group due to the “shielding effect” caused by the addition of new energy levels. The inner energy levels act in a way to shield the attractive charges of the nucleus for the outer electrons. Periodic Table Geography The horizontal rows of the periodic table are called PERIODS. The elements in any group of the periodic table have similar physical and chemical properties! The vertical columns of the periodic table are called GROUPS, or FAMILIES. Alkali Metals Alkaline Earth Metals Transition Metals These elements are also called the rare-earth elements. InnerTransition Metals Halogens Noble Gases S block P block D block F block Group Properties of Some Main Group Elements Alkali metals Halogens Alkaline Earth Metals Halogens 2 Noble Gases Braniac Alkali Metals