* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download C. trachomatis
Eradication of infectious diseases wikipedia , lookup
Yellow fever wikipedia , lookup
Lyme disease wikipedia , lookup
Anaerobic infection wikipedia , lookup
Hepatitis C wikipedia , lookup
West Nile fever wikipedia , lookup
Sarcocystis wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Brucellosis wikipedia , lookup
Chagas disease wikipedia , lookup
Trichinosis wikipedia , lookup
Marburg virus disease wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Mycoplasma pneumoniae wikipedia , lookup
Typhoid fever wikipedia , lookup
Dirofilaria immitis wikipedia , lookup
Hepatitis B wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Onchocerciasis wikipedia , lookup
Neonatal infection wikipedia , lookup
Neisseria meningitidis wikipedia , lookup
Visceral leishmaniasis wikipedia , lookup
Oesophagostomum wikipedia , lookup
Lymphocytic choriomeningitis wikipedia , lookup
Schistosomiasis wikipedia , lookup
Leptospirosis wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
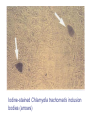
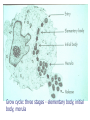
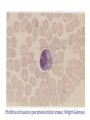
Chapter 47 Chlamydiaceae (披衣菌, 衣源體 ) Yu Chun-Keung DVM, PhD Department of Microbiology and Immunology Family Chlamydiaceae Genus Chlamydia: C. trachomatis (砂眼披衣菌) Genus Chlamydophilia: C. pneumoniae (肺炎披衣菌) C. psittaci (鸚鵡熱披衣菌) Chlamydiaceae Obligate intracellular organisms, were once considered virus. Possess inner and outer membranes Contain DNA and RNA Possess ribosomes Synthesize proteins, nucleic acid, and lipids, but cannot synthesize ATP. Respond to wide-spectrum antibiotics, but not to penicillin (lack peptidoglycan) Unique development cycle Two morphological distinct forms in cytoplasmic phagosome: (1) elementary body (300-400 nm), resistant to harsh environmental factor; bind to receptors of host cells and stimulate uptake; metabolically inactive but infectious, (2) reticulate body (800-1000 nm), reproductive form, divide by binary fission, noninfectious. Growth cycle EBs → host cells → prevent phagolysosomal fusion (if outer membrane is intact) → EBs reorganize into RBs within 6-8h → synthesis DNA, RNA, proteins (but not ATP, so-called energy parasite) → RBs replicate by binary fission for 18-24h → RBs reorganize into EBs 48-72h → cell rupture and release of EB. Histologic stains can detect phagosome with accumulated RBs (inclusion) 1. Chlamydia trachomatis (砂眼披衣菌) Infections only occur in humans Two biovars (trachoma and LGV) and 19 serotypes (antigen differences in MOMP) Biovars Serotypes Trachoma A to C D to K LGV L1 to L3 Disease Trachoma Urethritis, cervicitis Inclusion conjunctivitis Neonatal conjunctivitis Infant pneumonia Lymphogranuloma venereum Pathogenesis EBs enter the body via minute abrasions and lacerations Trachoma biovars primarily infect nonciliated columnar, cuboidal, or transitional epithelial cells (urethra, endocervix, endometrium, fallopian tube, anorectum, respiratory tract, conjunctiva) LGV biovars replicate in mononuclear phagocytes in lymphatic system (formation of granuloma, abscesses, or sinus tracts in LN draining the site of primary infection) Pathogenesis Destruct cells during replication Infection stimulates a severe inflammatory response (neutrophils, lymphocytes and plasma cells). No long-lasting immunity after infection Re-infection induces a vigorous inflammatory response with subsequent tissue damage (blindness and sterility). Trachoma (砂眼) A chronic suppurative eye disease caused by serotypes A,B,Ba,C. Follicular conjunctivitis →scar →corneal ulceration →pannus formation (翳, 音易) (invasion of vessels into the cornea,) →blindness Endemic in the Middle East, North Africa, and India (dry and sandy regions); predominantly in children. Leading global causes of blindness (500 million infected, 7 to 9 million blinded). Transmission: eye-to-eye by droplet, hands, contaminated clothing, flies. Urogenital infections Venereal infections caused by serotypes of D to K. The most common sexually transmitted bacterial disease in U.S. 2.8 million new cases annually, largely in males (50 million worldwide). In women: 80% asymptomatic; bartholinitis, cervicitis, pelvic inflammatory disease, which can lead to sterility and ectopic pregnancy. In men: 25% asymptomatic; nongonococcal urethritis (NGU) Nongonococcal urethritis 1. Mild 2. Slow and prolonged 3. Dysuria is mild 4. Urethral discharge is clear or white, thin and mucoid Gonorrhea 1. 2. 3. 4. Severe Acute Severe dysuria Purulent discharge Nongonococcal Urethritis (NGU) Urethritis caused by pathogens other than gonococcus C. trachomatis (35-50% of cases) Ureaplasma urealyticum (10-30% of cases) Mycoplasma hominis Gardnerella vaginalis Trichomonas vaginalis Candida albicans Herpesvirus hominis (?) Cytomegalovirus (?) Dual infections with both C. trachomatis and Neisseria gonorrhoeae are common. 淋菌後尿道炎: symptoms of chlamydial infection develop after successful treatment of gonorrhea because the incubation period is longer and the use of β–lactum antibiotics to treat gonorrhea would be ineffective against C. trachomatis Adult Inclusion Conjunctivitis (成人包涵性結膜炎) Acute follicular conjunctivitis with mucopurulent discharge Mostly occur in sexually active adults (18-30 yr) with genital infection with serotypes A, B, Ba, D to K. Auto-inoculation, oral-genital contact Newborn Inclusion Conjunctivitis 25% infants acquired from mothers with active genital infections Long (>12 months) disease course if untreated and are at risk for C. trachomatis pneumonia Infant Pneumonia A diffuse interstitial pneumonia Occur in 10-20% infants that exposed to the pathogen at birth Rhinitis → staccato cough (afebrile) Reiter’s syndrome: Urethritis, conjunctivitis, polyarthritis Usually occurs in young white man 50-65% patients have chlamydial genital infection at the onset of arthritis Lymphogranuloma venereum (LGV) 花柳性淋巴肉芽腫 A chronic sexually transmitted disease caused by C. trachomatis L1, L2, L2a, L3. More common in men, with male homosexuals being the major reservoir. Small, painless lesions at site of infection (genitalia). Fever, headache, myalgia. Swelling of regional lymph nodes (inguinal nodes), painful buboes (橫瘻), rupture. Proctitis is common in women. Resolve spontaneously or progress to ulceration or genital elephantiasis (象皮病). Bubonic plague – Inguinal buboes with edema Lab diagnosis Symptomatic infections are easier to diagnosis than asymptomatic infections Cytology – Giemsa-stained cell scrapings Quality of the specimen is important. Specimens must be obtained from the involved site; pus or exudate is inadequate. Insensitive, nonspecific Culture – HeLa, MaCoy, Hep-2 cells; iodine strain to detect inclusions; the most specific methods for diagnosis. Iodine-stained Chlamydia trachomatis inclusion bodies (arrows) Chlamydial urethritis (elementary bodies in direct smear of urethral cell, fluorescein antibody stain) Lab diagnosis Nucleic acid amplification tests (NAATs) – test of choice for lab diagnosis of C. trachomatis infection Serologic tests – limited value for adult urogenital infections; good for LGV. CF test or EIAs: genus-specific LPS, fourfold increase or >1:256 MIF test: species- and serovar-specific antigen (MOMPs) 2. Chlamydophilia pneumoniae (肺炎披衣菌) Was first isolated from the conjunctiva of a child in Taiwan - TWAR stain. An important cause of bronchitis, pneumonia and sinusitis. Infection is common, especially in adults and transmitted person-to-person by respiratory secretions. Clinical disease Most infections are asymptomatic or mild - persistent cough. Can’t be differentiated with other atypical pneumonia - M. pneumoniae, Legionella pneumophila, and respiratory viruses. Detected in atherosclerotic lesions in blood vessels. However, the role in the development of atherosclerosis is not clear. (Koch’s postulate) Lab diagnosis Diagnosis is difficult Do not grow in cell lines NAATs are OK for use. Complement fixation test (not specific) or MIF test (specific) 3. Chlamydophilia psittaci (鸚鵡熱披衣菌) Caused Psittacosis (parrot fever). The natural reservoir is any species of birds (Ornithosis,飼鳥病) Veterinarians, zookeepers, pet shop workers, employees of poultry industry. Pathogenesis Inhalation of dried bird excrement, urine, or respiratory secretions; person-to-person transmission is rare. Bacteria spread to and multiply in reticuloendothelial cells of liver and spleen necrosis Disseminate to lung and other organs via circulation Lmphocytic inflammation in lung, edema, necrosis, mucous plugs cyanosis and anoxia C. psittaci has three forms of infection Asymptomatic infection Transient flu-like illness: high fever, headache, chills, myalgia Serious pneumonia: non-productive cough, rales, CNS involvement is common, carditis, hepatomegaly, splenomegaly Diagnosis and treatment for C. psittaci Diagnosis: complement fixation test with group antigen, fourfold rise in specific antibody Treatment: tetracyclines or macrolides Treat birds with chlortetracycline HCl for 45 days. C. trachomatis C. pneumoniae C. psittaci Disease severe Glycogen absent No staining with iodine Disease mild and chronic Glycogen in inclusions Inclusions can be stained with iodine Susceptible to sulfonamides Sulfonamide resistant Chapter 45 Rickettsia and Orientia Chapter 46 Ehrlichia, Anaplasma, Coxiella Rickettsia Howard Ricketts Ehrlichia Paul Ehrlich Coxiella Harold Cox (Historically classified in Rickettsiaceae) Order Rickettsiales Family Rickettsiaceae Genena Rickettsia Orientia Family Anaplasmataceae Genena Ehrlichia Anaplasma Neorickettsia Wolbachia Chapter 45 Rickettsia and Orientia General characteristics G(-) bacilli, obligate intracellular parasites. Were thought to be virus: small (0.3x1m), 800 genes. True bacteria: DNA+RNA, binary fission, sensitive to antibiotics, use host cell ATP. Maintain in animal and arthropod reservoirs. Transmitted to humans by arthropod vectors (ticks, mites, lice, fleas), and maintained in arthropod hosts by transovarian transmission. Humans are accidental hosts: acquired by arthropod bite or contact of arthropod excreta with abraded skin. The distribution of rickettsial diseases is determined by the distribution of the arthropod host/vector. Pathogenesis Rickettsia (also Ehrlichia) is unstable and die quickly outside host cells. Coxiella highly resistant to desiccation, remain viable in environment for months to years. No toxins, no immunopathology Rickettsia replicate in endothelial cells, cause cell damage and blood leakage, skin rash, microthrombi, focal ischemia, hemorrhage. Hypovolemia, hypoproteinemia, reduced perfusion, organ failure. After phagocytosis Rickettsia and Orientia: degrade phagosome membrane by producing phospholipase; multiply in cytoplasm Ehrlichia: multiply in cytoplasmic vacuoles (= phagosomes) Coxiella: multiply in phagolysosome Spotted fever group of Rickettsia and Orientia: release during infection Typhus group of Rickettsia: release after cell lysis Important Rickettsial Diseases Spotted fever group R. rickettsii R. akari RMSF (>90%) Rickettsialpox (100%) Typhus group R. prowazekii R. typhi Epidemic typhus (40-80%) Murine typhus (50%) Scrub typhus group O. tsutsugamushi Scrub typhus (<50%) (Parentheses: % of rash, 紅斑) Spotted fever 斑疹熱 Have a restricted geographic distribution; Rocky mountain spotted fever (RMSF) is the prototype of the group, caused by R. rickettsii. Organisms are maintained in hard ticks (wood tick and dog tick) by transovarian transmission. Transmitted to humans by ticks (need 24-48h to establish infection). High fever, chills, headache, skin rash (>90%, extremities to trunk) GI symptoms, respiratory failure, encephalitis, renal failure. Diagnosis is urgent, because the prognosis depends on the duration of illness. (identify key clinical signs – rash) Culture: tissue culture or embryonated eggs (danger) Microscopy: Giemsa stain; FA for biopsy tissue specimens (rapid and specific) Serology: Microimmunofluorescence (MIF), detect antibodies against MOMP and LPS antigens Molecular diagnosis: PCR, not speciesspecific Prevention/Control: Tetracyclines (e.g., doxycline) No vaccine Prevent tick bites (can survive for as long as 4 years without feeding) Epidemic (louse-borne) typhus 流行性(蝨型)斑疹傷寒 R. prowazekii transmits from man to man by human head and body lice. Humans are the primary reservoir (lice die 2 to 3 wk after infection). Epidemics occur among people living in crowded, unsanitary condition - war, famine, or natural disaster. High fever, severe headache, chills, followed by a generalized skin rash; complications: myocarditis and CNS involvement. O Brill-Zinsser Disease A recrudescent form of epidemic typhus arising years after the initial attack. Diagnosis: MIF test T/P/C: Tetracyclines, Chloramphenicol Louse-control Formaldehyde-inactivated vaccine Endemic (murine) typhus 地方性(鼠類)斑疹傷寒 R. typhi transmits to man from rodents reservoir hosts by rat flea. Endemic all over the world, primarily in warm, humid areas. Fever, severe headache, chills, skin rash (50%) on chest and abdomen o Diagnosis: IFA test T/P/C: Tetracyclines, doxycycline, Chloramphenicol Pest control No vaccine Scrub typhus 叢林斑疹傷寒 A rickettsial disease caused by Orientia tsutsugamushi (恙蟲病立克次體菌) Transmitted to humans by red mites (chiggers) Organisms are maintained in mites by transovarian transmission. Endemic in eastern Asia, Australia, and Japan. Fever, severe headache, skin rash (<50%), spread centrifugally to extremities. Generalized lymphadenopathy, splenomegaly, CNS complication, heart failure O 別來無恙? 歲亦無恙耶? 民亦無恙耶? 王亦無恙耶? <國策>「齊策‧四」 T/P/C: Prompt treatment with tetracyclines, doxycycline, chloramphenicol Avoid exposure to chiggers No vaccine Chapter 46 Ehrlichia, Anaplasma, Coxiella Ehrlichia and Anaplasma Infections of hematopoietic cells Intracellular bacteria that lodge in phagosomes of mononuclear and granulocytic phagocytes, but not RBC. multiply in phagosomes = morulae Grow cycle: three stages - elementary body, initial body, morula Ehrlichia inclusions (peripheral blood smear, Wright-Giemsa) Clinical diseases 1. Human monocytic ehrlichiosis E. chaffeensis : infect blood monocytes and mononuclear phagocytes in tissues and organs Vector - Lone Star tick Reservoir - white-tailed deer, domestic dogs 2. Canine granulocytic ehrlichiosis E. ewingii Vector - Lone Star tick Reservoir -white-tailed deer, domestic dogs. 3. Human anaplasmosis Anaplasma phagocytophilium : infect bone marrow myeloid cell (i.e., neutrophils) Vector - Ixodes ticks Reservoir - small mammals Clinical disease Fever, headache, malaise, leukopenia, thrombocytopenia Skin rash (10 to 40%) 50% patients require hospitalization, 1 to 3% mortality T/P/C: Diagnosis is urgent. Prompt treatment with doxycycline No vaccine Avoid tick-infested areas Coxiella burnetii 蒲奈氏科克斯菌 Biologically and genomically distinct from Rickettsia; more closely related to Legionella and Francisella. Obligate intracellular pathogen Multiply in phagolysosome Epidemiology Can infect mammals, birds, and ticks Primary reservoirs: farm animals, cats, dogs, rabbits Ticks are vector for disease in animals but not in humans Epidemiology High concentrations of bacteria are present in placenta of infected livestock. Spores are able to survive in nature under dry environmental conditions for months. Transmit to man by the respiratory route from contaminated soil, not from arthropod vector. Those handling pregnant or lactating cows or sheep, drinking unpasteurized milk, or working in slaughter-houses are at highest risk. Pathogenesis Target tissue is the lung, proliferate in phagolysosomes of infected cells, then disseminate to other organs Undergo antigenic variation (cell wall LPS): phase I antigen: LPS with a complex carbohydrate, can block antibody binding; infectious form phase II antigen: modified LPS, expose surface proteins to antibody, less infectious form Formation of immune complex: cause of signs and symptoms Q fever Most infections are mild or asymptomatic Acute disease: Pneumonia - high fever, severe headache, chill, myalgias, resemble “atypical pneumonia” Granulomatous hepatitis, hepatosplenomegaly, Chronic disease: subacute endocarditis with long incubation period and poor prognosis Diagnosis Serologic tests (IFA, ELISA, CF) Acute Q fever: IgM and IgG are developed against phase II antigen. Chronic Q fever: antibodies against both phase I and II antigens are elicited. (phase I antigen: weak antigenic) T/P/C: Doxycycline for prolonged period Vaccine is available (single dose with no booster immunization for uninfected people) 96.5.14