* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Rickettsia and Orientia
Survey
Document related concepts
Rheumatic fever wikipedia , lookup
Sociality and disease transmission wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
2001 United Kingdom foot-and-mouth outbreak wikipedia , lookup
Sjögren syndrome wikipedia , lookup
Transmission (medicine) wikipedia , lookup
Transcript
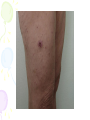
Rickettsia and Orientia • Rickettsia (Rickettsia and Orientia) •Ehrlichia (Ehrlichia and Anaplasma) •small (0.3 × 1 to 2 μm) •stained poorly with the Gram stain •grew only in the cytoplasm of eukaryotic cells Rickettsia and Orientia • structurally similar to gram-negative rods • contain DNA, ribonucleic acid (RNA), and enzymes and ribosomes • multiply by binary fission • inhibited by antibiotics Rickettsia and Orientia • maintained in animal and arthropod reservoirs • transmitted by arthropod vectors (e.g., ticks, mites, lice, fleas) • humans are accidental hosts • spotted fever group and the typhus group Rickettsia and Orientia • Organism Human Disease Spotted Fever Group R. Rickettsii Rocky Mountain spo.f. R. Africae African tick bite f. R. Akari Rickettsialpox R. Australis Australian tick typhus R. Conorii Mediterranean spo. f. R.japonica Japanese spotted f. R. Sibirica Siberian tick typhus Typhus group R. Prowazekii Epidemic R.typhi Endemic Scrub typhus group O.tsutsugamishi Scrub typhus Distribution Western hem Eastern-Sou Africa Worldwid Australia Mediterranean Japan Siberia, Mongolia, Worldwide Worldwide Asia, Ocenia Rickettsia and Orientia Bite of brown dog tickRhipicephalus sanguineus Rickettsia and Orientia • peptidoglycan layer is minimal • LPS has only weak endotoxin activity • binary fission is slow Rickettsia and Orientia • No toxins • No host immune response • R. rickettsii is most common rickettsial pathogen in United States • Hard ticks are the primary reservoirs and vectors (Dermacentor) • Transmission requires prolonged contact (24 to 48 hours) • Distribution in Western hemisphere • Disease is most common April through October Rickettsia and Orientia • 2-14 days • Painless tick bite • High fever, headache, fever, chills • Rash macular to petechial • First extremities and then trunk • GIS symptoms, respiratory failure • Encephalitis, renal failure Rickettsia and Orientia • Tissue culture and embrynonated eggs • Microscopy • Serology • PCR Rickettsia and Orientia • Tetracycline • Fluroquinolones Rickettsia and Orientia • Epidemic typhus • Louse-borne typhus • Humans are the primary reservoir • Replicates in endothelial cells with resulting vasculitis Rickettsia and Orientia • Humans are the primary reservoir, with personto-person transmission by louse vector • It is believed that sporadic disease is spread from squirrels to humans via squirrel fleas • Recrudescent disease can develop years after initial infection • People at greatest risk are those living in crowded, unsanitary conditions • Disease is worldwide, with most infections in Central and South America and Africa • Sporadic disease is seen in the eastern United States Rickettsia and Orientia • 2- to 30-day incubation period • nonspecific symptoms • less than 40% of the patients had a petechial or macular rash • myocarditis and central nervous system dysfunction • Brill-Zinsser disease-milder Rickettsia and Orientia • MIF test is the diagnostic method of choice • Tetracyclines and chloramphenicol • Formaldehyde-inactivated typhus vaccine Rickettsia typhi • • • • • • • • Endemic or murine typhus worldwide Rodents are the primary reservoir, Rat flea (Xenopsylla cheopis) is the principal vector 7 to 14 days A rash develops Typically restricted to the chest and abdomen Indirect fluorescent assay Orientia tsutsugamushi • Scrub typhus • Mites • Asia, Oceania • 6-18 days • Sudden onset • Maculo-papular rash • LAP, SM • Tetracycline, chloramphenicol Ehrlichia, Anaplasma, and Coxiella • Anaplasmataceae: Anaplasma, Ehrlichia, Neorickettsia, and Wolbachia • survival within a cytoplasmic vacuole in the infected arthropod or mammalian cell • infection of hematopoietic cells Ehrlichia, Anaplasma, and Coxiella Multiple morulae of Ehrlichia canis in DH82 tissue culture cells Ehrlichia, Anaplasma, and Coxiella • Small, intracellular bacteria • Stain poorly with Gram stainReplicates in phagosome of infected cells • Intracellular growth protects bacteria from immune clearance • Able to prevent fusion of phagosome with lysosome of monocytes or granulocytes • Initiates inflammatory response that contributes to pathology Ehrlichia, Anaplasma, and Coxiella • Depending on the species of Ehrlichia, important reservoirs are white-tailed deer, white-footed mouse, etc • Ticks are important vectors, but transovarian transmission in inefficient • Disease in United States is most common in the Atlantic states; northern, central, and southern Midwest states; and northern California • People at greatest risk are those exposed to ticks in the endemic areas • Disease is most common from April to October Ehrlichia, Anaplasma, and Coxiella • Human monocytic ehrlichiosis is caused by E. chaffeensis • 1 to 3 weeks after a tick bite, patients develop a flulike illness with fever, headache, and myalgias • Gastrointestinal symptoms develop in fewer than half the infected patients • late-onset rash develops in 30% to 40% of patients • Leukopenia, thrombocytopenia, and elevated serum transaminases Ehrlichia, Anaplasma, and Coxiella • Canine Granulocytic Ehrlichiosis E. ewingii • Human anaplasmosis, A. phagocytophilum • More than half the infected patients require hospitalization, and severe complications are common • Mortality is rare Ehrlichia, Anaplasma, and Coxiella • Giemsa-stained preparations of peripheral blood should be performed, morulae diagnostic • PCR • Tetracycline, rifampin pregnant women • Vaccines are not available Coxiella burnetii • more closely related to Legionella and Francisella • Q fever, which may be asymptomatic in humans and develops either acutely or as a chronic infection • small, pleomorphic coccobacillus (0.2 to 0.7 μm) • The small replicating cells will mature to large-cell variants, which then evolve to stable spores Coxiella burnetii • inhalation of airborne particles • more by the environment • Coxiella proliferate in the respiratory tract and then disseminate to other organs • pneumonia and granulomatous hepatitis • most chronic infections manifest as endocarditis Coxiella burnetii • antigenic variation • C. burnetii is extremely stable in harsh environmental conditions • Many reservoirs, including mammals, birds, and ticks • Most human infections associated with contact with infected cattle, sheep, goats, dogs, and cats • Most disease acquired through inhalation; possible disease from consumption of contaminated milk; ticks are not an important vector for human disease • Worldwide distribution • No seasonal incidence Coxiella burnetii • Acute diseases include influenza-like syndrome, atypical pneumonia, hepatitis, pericarditis, myocarditis, meningoencephalitis • Chronic diseases include endocarditis, hepatitis, pulmonary disease, and infection of pregnant women Coxiella burnetii • most common presentation of chronic Q fever is subacute endocarditis • culture (not commonly performed), polymerase chain reaction (PCR), or by specific serologic tests • serology is the most commonly used diagnostic test • Tetracycline • combination of drugs, such as rifampin and either doxycycline or trimethoprimsulfamethoxazole