* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Congenital Heart Disease
Management of acute coronary syndrome wikipedia , lookup
Heart failure wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Coronary artery disease wikipedia , lookup
Myocardial infarction wikipedia , lookup
Cardiac surgery wikipedia , lookup
Artificial heart valve wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Aortic stenosis wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Atrial septal defect wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
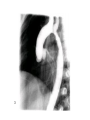
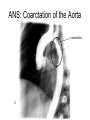
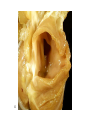
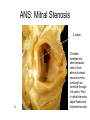
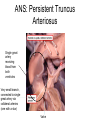
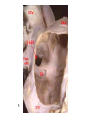
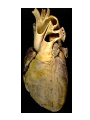
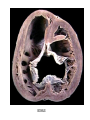
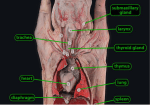
Congenital Heart Disease Lab Module December 17, 2009 Instructions • From what you have learned in the lecture, try to diagnose the congenital cardiac anomaly that is being illustrated in each photograph • Try to orient the specimen or photo first, then identify landmarks, then look for the defect • Good luck! Septal defect in membranous part 1 ANS: Perimembranous VSD • Type that occurs in about 90% of VSD cases. (Robbins) • Involves region of membranous septum (as opposed to infundibular VSD, which lies below the pulmonary valve or within the muscular septum) (Robbins) • Located in the left ventricle outflow tract beneath the aortic valve (emedicine) • Defects may extend into adjacent portions of the ventricular septum. (emedicine) • Perimembranous VSD is caused by failure of the endocardial cushions, the conotruncal ridges, and the muscular septum to fuse at a single point in space. (emedicine) • Closes spontaneously in most cases. (emedicine) ANS: Coarctation of the Aorta branches of the aorta Constriction 2 This image is of an aortic arch; the aorta has been opened up. Coarctation of the Aorta • Structural anomaly • Constriction or narrowing of the aorta • Two classic forms: – With PDA (often symptomatic in early childhood) – Without PDA (usually asymptomatic children) 3 ANS: Coarctation of the Aorta constriction 3 4 ANS: Mitral Stenosis 2 cusps 4 Chordae tendinae not seen because view is from above (kunwari nasa atrium ka tumitingin sa ventricle through the valve). Pero in mitral stenosis, dapat fused and thickened na sila. ANS: Mitral Stenosis • Failure of mitral valve to open completely, thereby impeding forward flow to the left ventricle (Robbins) • Cardinal anatomic changes: – Leaflet thickening – Commisural fusion and shortening – Thickening and fusion of the tendinous chords • Fibrous bridging and calcification usually result in “fish mouth” or “buttonhole” stenoses. • NOTE: The trick daw when presented with a picture of a valve is to first orient the picture in relation to the ventricles and/or atria. Then count the cusps. Mitral valve – bicuspid (2 cusps); Tricuspid – tricuspid duh haha (3 cusps). Semilunar valves look alike – 3 fibrous leaflets. 7 ANS: Persistent Truncus Arteriosus Single great artery receiving blood from both ventricles Very small branch, connected to single great artery via collateral arteries (one with a star) Valve ANS: Persistent Truncus Arteriorsus • Arises from a developmental failure of separation of the embryologic truncus arteriosus into the aorta and the pulmonary artery. • Results in a single great artery that receives blood from both ventricles and gives rise to the systemic, pulmonary, and coronary circulations. • Because there is an associated VSD and mixing of blood from the right and left ventricles, PTA produces systemic cyanosis as well as an increased pulmonary blood flow, with the danger of irreversible pulmonary hypertension. 8 ANS: Tetralogy of Fallot • There are four cardinal features of TOF: 1. VSD 2. Obstruction of the right ventricular outflow tract (subpulmonary stenosis) 3. An aorta that overrides the VSD. 4. Right ventricular hypertrophy. Morphology of the heart Often enlarged and may be “boot-shaped as a result of the marked right ventricular hypertrophy, particularly the apical region • The VSD is usually large. • The aortic valve forms the superior border of the VSD, thereby overriding the defect and both ventricular chambers • Obstruction to the RV flow is most often due to narrowing of the infundibulum (subpulmonic stenosis) but can be accompanied by pulmonary valvular stenosis • If the subpulmonary stenosis is mild, the abnormality resembles an isolated VSD, and the shunt may be left-to-right, without cyanosis (so-called pink tetralogy) • As the obstruction increases in severity, there is a commensurately greater resistance to right ventricular outflow. As right-sided pressures approach or exceed left-sided pressures, right-to-left shunting develops, producing cyanosis (Classic TOF). • The subpulmonary stenosis, however, protects the pulmonary vasculature from pressure overload, and right ventricular failure is rare because the right ventricle is decompressed by the shunting of blood into the left ventricle and the aorta. 9 ANS: Anomalous Pulmonary Venous Connection • Results when the pulmonary veins fail to directly join the left atrium. • Results embryologically when the common pulmonary vein fails to develop or becomes atretic. • Either patent foramen ovale or and ASD is always present, allowing pulmonary venous blood to enter the left atrium. • Cyanosis may be present as a result of mixing of well-oxygenated and poorly oxygenated blood at the site of the anomalous pulmonary venous connection and the large right-to left shunting through ASD. • Consequences: – Volume and pressure hypertrophy and dilatation of the right side of the heart – Dilatation of the pulmonary trunk . – Left atrium is hypoplastic – Left ventricle is usually normal in size ANS: Patent Ductus Arteriosus • Also called Persistent Ductus Arteriosus. • Results when the ductus arteriosus, an essential fetal structure that normally spontaneously closes, remains open after birth. • Occurs as an isolated anomaly 90% of the time. The remainder are most often associated with VSD, coarctation of the aorta, or pulmonary or aortic valve stenosis • Produces a characteristic continuous harsh murmur, described as “machinery-like.” • Usually asymptomatic at birth. • There is no cyanosis, but eventually the additional volume and pressure overload produce obstructive changes in small pulmonary arteries, leading to reversal of flow and its associated consequences. • Preservation of ductal patency (by administering prostaglandin E) assumes great importance in survival of infants with various congenital malformations that obstruct the pulmonary or systemic outflow tracts (e.g.patients with aortic valve atresia). 10 ANS: Persistent Truncus Arteriorsus • Arises from a developmental failure of separation of the embryologic truncus arteriosus into the aorta and the pulmonary artery. • Results in a single great artery that receives blood from booth ventricles and gives rise to the systemic, pulmonary, and coronary circulations. • • Because there is an associated VSD and mixing of blood from the right and left ventricles, PTA produces systemic canosis as well as an increased pulmonary blood flow, with the danger or irreversible pulmonary hypertension. BONUS ANS: Atrioventricular Septal Defect • Results from the embryologic failure of the superior and inferior endocardial cushions of the AV canal to fuse adequately. • The consequence is incomplete closure of the AV septum and malformation of the tricuspid and mitral valves. • Two Most Common Forms: – Partial AVSD (consisting of a primum ASD and a cleft anterior mitral leaflet, causing mitral insufficiency) – Complete AVSD (consisting of a large combined AV septal defect and a large common AV valve—essentially a hole in the center of the heart. • In complete form, all four cardiac chambers freely communicate, inducing volume hypertrophy of each. • More than 1/3 of all patients with a complete AVSD have Down Syndrome. Surgical repair is possible.