* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download the PowerPoint
Heart failure wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Electrocardiography wikipedia , lookup
Coronary artery disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Cardiac arrest wikipedia , lookup
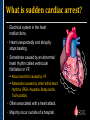
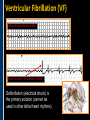
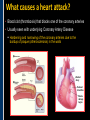
SUDDEN CARDIAC ARREST A Community Presentation on the ALPS Study PORTLAND RESUSCITATION OUTCOMES CONSORTIUM What is this forum about? • We want your opinion on research that might involve you or a family member. We are studying the use of heart rhythm medications in cardiac arrest. This will be done with an Exception From Informed Consent. What is Exception from Informed Consent (EFIC) • A federal regulation (21 CFR 50.24) allows studies that meet the following rules to use this exception: Patient’s lives must be at risk. Available treatments are not satisfactory. Patients are unable to give consent. The possible risks are reasonable. Being in the research study could help patients (increased survival). It would not be possible to do the research practically without this exception. EFIC Regulations • Collect input and opinions from the community about the possible research. Tell the community about the research (public disclosure) Find out what people think (community consultation) • Patients who are in this study will need immediate treatment. Without intervention, patients in cardiac arrest will quickly die. • We cannot collect informed consent before starting treatment because: Patients in cardiac arrest are unconscious. They don’t have a pulse and not able to give us permission. Treatment must start immediately, and the next of kin may not be present, or may be too upset to understand an the research study. “Opt-Out” Option • An option for community members. • A “No Study” bracelet will be sent to those who ask for one by calling 503-494-8083 or email [email protected]. Notification & Consent • The study interventions are completed upon patient arrival to the hospital. • We will ask for consent from patients to review medical records related to the current hospitalization only. • Taking part in this study is very brief. People will be allowed to leave this study as soon as they are able to say they want to stop. Public Health Problem • Cardiovascular disease (CVD) is the number one health threat to most adult Americans. • Each year, 1.25 million people experience an acute myocardial infarction (MI) or “heart attack.” • Approximately 300,000 to 350,000 persons die from out-ofhospital cardiac arrest (“sudden death”) each year in North America. Survival rate remains poor (less than 8% nationally) Sudden death can happen shortly after a person start to have heart attack symptoms It’s important to note that a heart attack is NOT the same thing as a cardiac arrest! Cardiac arrest can be a devastating complication of a heart attack. This study focuses on cardiac arrest specifically. Heart Attack – Why call 9-1-1 • Early treatment by Emergency Medical Services (EMS) providers* Oxygen Heart monitoring Medications Electrocardiogram (“EKG”) • Getting patients to treatment quickly (Rapid transport to appropriate facility). • Treatment of problems and complications. Sudden cardiac arrest Low blood pressure, heart failure *Local fire departments and ambulance services staffed with EMTs and paramedics. What is sudden cardiac arrest? • Electrical system in the heart malfunctions. • Heart unexpectedly and abruptly stops beating. • Sometimes caused by an abnormal heart rhythm called ventricular fibrillation or VF. About one-third caused by VF. Remainder caused by other lethal heart rhythms (PEA, Asystole, Bradycardia, Tachycardia). • Often associated with a heart attack. • Majority occur outside of a hospital. Ventricular Fibrillation (VF) What VF looks like on an EKG Shock “converts” VF to better rhythm Defibrillation (electrical shock) is the primary solution (cannot be used in other lethal heart rhythms) Importance of Early Defibrillation % Success 100 90 Chances of success decrease 7–10% each minute 80 70 60 50 40 30 20 10 0 0 1 2 3 4 5 6 7 Time to Defibrillation (minutes) 8 9 American Heart Association (AHA) “Chain of Survival” • Communities with the following things in place tend to have the best rates of survival: Understanding that emergency services are needed and calling 9-1-1 immediately. Early CPR, especially with quality chest compressions Rapid defibrillation (an electrical shock to the heart) Effective paramedics (advanced life support ) Follow up care (post-cardiac arrest care) The “Chain of Survival” The ALPS Trial • ALPS: Amiodarone, Lidocaine, Placebo, Study • Amiodarone and Lidocaine are medications currently used by paramedics to stabilize the heart (referred to as heart rhythm medications). • Normally given if VF continues or recurs after the first defibrillation The ALPS Trial • We do not know which of the two medications is the most effective. • Or whether they are effective at all. • ALPS will attempt to find out which is better, or if neither (the placebo, normal saline) is better. Preliminary Trials • Two prior trials: Amiodarone may be better than Lidocaine, as well as no drug therapy (placebo). Both studies looked at how many people were admitted to the hospital after cardiac arrest. Neither study had enough patients to see how many patients survived to hospital discharge. • Both medications are currently used in our EMS system. Both are used as one of the main treatments. • Both medications could be harmful. We do not know how many patients survive to hospital discharge. False hope The use of these drugs may stop people from receiving other more effective treatments. How will the trial work? • EMS services will have a kit with three syringes. • The kit will contain either Amiodarone, Lidocaine or saline in each of the three syringes. • The paramedics will not know what is contained in the syringes. • In cases of cardiac arrest, where VF recurs after an initial defibrillation, they will use the syringes in the study kit Drug Kit Design Three (3) identical (blinded) syringes SYRINGE # AMIODARONE KIT LIDOCAINE KIT PLACEBO KIT 1 Amiodarone 150 mg (3 cc) Lidocaine 60 mg (3 cc) Placebo (3 cc) 2 Amiodarone 150 mg (3 cc) Lidocaine 60 mg (3 cc) Placebo (3 cc) 3 Amiodarone 150 mg (3 cc) Lidocaine 60 mg (3 cc) Placebo (3 cc) Why is there a placebo? • A recent study from Norway showed that there was NO difference in survival from cardiac arrest when individuals were given intravenous medications (including heart rhythm agents) when compared to no medications outside the hospital. • Also, medications can have side effects that could cause problems and actually make the effects of cardiac arrest worse. ALPS Study Outcome Measures • Primary Survival to hospital discharge • Secondary Survival to hospital discharge with good function Potential Benefits • Paramedics taking part in this study will receive extra training. Patients may benefit from this extra training (the Hawthorne effect). • Results would change how we treat/resuscitate patients worldwide. Who will be included in the study? • Patients that will be included: Adult patients in cardiac arrest in whom VF recurs after the first defibrillation. • Patients that will be excluded: Known pregnant women. Children under the age of 18 years. Prisoners. Patients wearing a “No Study” bracelet. Patients with Do Not Resuscitate (DNR) orders ALPS Patient Safety Monitoring • The study will be monitored by: Data Safety Monitoring Board (DSMB)—an independent group Institutional Review Board (IRB) Food & Drug Administration (FDA) National Institutes of Health (NIH) Potential Adverse Events • Amiodarone: Slow heart rate, low blood pressure, vein irritation • Lidocaine: Seizures, slow heart rate, low blood pressure • These will be watched and tracked and reported to the FDA and DSMB Our Ultimate Goal To find the best treatment methods for managing cardiac arrest, in order to save more lives! Questions? Do you have any concerns about this proposed research study? For more information, visit our website at: www.ohsu.edu/emergency/roc Supplemental Slides To be used if questions about heart attacks come up in the discussion. What is a heart attack? • A heart attack is caused by the interruption of blood flow to the heart. • If this continues, heart muscle cells suffer injury and die. • Depending on how much heart muscle is damaged, disability or death can occur. • In some cases, a heart attack can result in cardiac arrest. • Other names for a heart attack: Acute Myocardial Infarction (AMI) Myocardial Infarction (MI) Coronary Thrombosis Coronary Occlusion What causes a heart attack? • Blood clot (thrombosis) that blocks one of the coronary arteries • Usually seen with underlying Coronary Artery Disease Hardening and narrowing of the coronary arteries due to the buildup of plaque (atherosclerosis) in the walls Blocked artery Reduced blood flow Damaged area Muscle damage begins Symptoms of a Heart Attack • Chest pain, discomfort, pressure, or squeezing • Upper-body pain or discomfort in one or both arms, back, shoulders, neck, jaw, or upper part of stomach • Shortness of breath • Breaking out in a cold sweat • Unusual or unexplained fatigue (tiredness), particularly in women (may be present for days) • Nausea/vomiting • Light-headedness or sudden dizziness A Heart Attack is an Emergency! • Time is critical! • Prompt treatment can reduce damage! • Early intervention can prevent death! CALL 9-1-1! Don’t drive yourself to the hospital!