* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Heart failure wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Myocardial infarction wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Coronary artery disease wikipedia , lookup
Aortic stenosis wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Cardiac surgery wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Atrial septal defect wikipedia , lookup
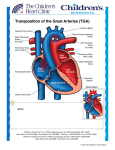
Dextro-Transposition of the great arteries wikipedia , lookup
D-TGA
DR.VINOD.G.V
TRANSPOSITION
Abnormal origin of the Aorta and Pulmonary Artery
from the ventricular complex
Atrioventricular concordance with ventriculo-
arterial discordance
Abnormal spatial relationship of the great arteries
Results in two circulations in parallel
Incidence & Prevalence
5% to 7% of all congenital cardiac malformations
The incidence is reported to range from 20.1 to
30.5/100,000 live births
strong (60%–70%) male preponderance
Embryology
Trunco conal malseptation Hypothesis
Normally related great arteries result from spiral
downgrowth of the truncoconal septum,
whereas TGA results from straight downgrowth
of the trunco conal septum.
The embryonic Aortic switch procedure
During development of the conotruncal
region, the pulmonary artery is related
to the left conus and the aorta to the
right conus
normal movement of the pulmonary
valve proceeds from posterior to anterior
on the left side in the interval between
30 and 34 days of age and is related to
normal development of the
subpulmonary infundibulum
During this same interval, the aortic
valve remains stationary, apparently
because of the normal lack of
development (or absorption) of the
subaortic infundibulum
abnormal growth and development of the
subaortic infundibulum and the absence of
growth of the subpulmonary infundibulum.
The aortic valve is protruded superiorly and
anteriorly by the development of the subaortic
infundibulum, placing it above the anterior right
ventricle .
Failure of development of the subpulmonary
infundibulum prevents the normal
morphogenetic movement of the pulmonary
valve from posterior to anterior and further
results in abnormal pulmonary to mitral valve
ring fibrous continuity
Anatomy
The common clinical type - situs solitus of the
atria, concordant AV and discordant
ventriculoarterial alignments -
complete TGA.
TGA {S,D,D} - TGA with situs solitus (S) of the
atria and viscera, usual (D) looping of the
ventricles and an anterior and rightward (D) aorta.
Great artery relationship
Situs solitus and intact ventricular septum - the
aortic root is directly anterior or anterior and to the
right of the pulmonary trunk in a slightly oblique
relationship
Less commonly, the aorta may be positioned
anterior and to the left or, rarely, posterior and to
the right of the pulmonary trunk.
Coronary Anatomy
The two aortic sinuses of Valsalva adjacent to the
aorticopulmonary septum that “face” the
pulmonary artery contain the ostia of the coronary
arteries in more than 99% of cases
Coronary anatomy
Usual-66.9
CX from RCA-16.1
Single RCA-3.9
Single LCA-1.7
Inverted-2.4
Intramural LCA-2.1
Other-1.6
SA node artery
Origin and proximal course of artery may be variable;
reaches the sinus node by the interatrial groove on the
anterior surface of the heart, occasionally with an
intramyocardial course in the anterosuperior rim of the
fossa ovalis.
can be damaged easily during balloon atrial
septostomy, during surgical septectomy or when this
portion of the septum is widely excised as in the
Mustard or Senning atrial switch operation.
Coexisting Anomalies
Nearly half of the hearts have no other anomaly except a
PFO or a PDA.
The VSD is the most frequent coexisting anomaly-40% to
45%.
- perimembranous (33%)
- inlet septum( 5%)
- muscular (27%)
- malalignment (30%)
- conal septal hypoplasia type (5%)
Malalignment VSD
anterior malalignment of the infundibular septum
is frequently associated with sub aortic stenosis,
aortic arch hypoplasia, coarctation ,complete
interruption of the aortic arch
Posterior (leftward) malalignment is associated
with varying degrees of LVOTO–subpulmonary
stenosis, annular hypoplasia or even pulmonary
valvar atresia
Subpulmonary Stenosis (25%)
Fixed
-Circumferrential fibrous membrane /diaphragm
- Fibromuscular ridge
- Herniating tricuspid leaflet tissue
- Anomalous MV septal attachments
- Tissue tags from membranous septum
Dynamic-associated with SAM
Subaortic Obstruction
Rightward and anterior displacement of the
infundibular septum
Associated aortic arch anomalies
- hypoplasia
- coarctation
- interruption
Asso. RV hypoplasia & tricuspid valve anomalies
TV anomalies
Straddling/overriding of chordae
Overriding of the tricuspid annulus
Abnormal chordal attatchments
Dysplasia
Accessory tissue
Double orifice
MV anomalies
Nearly 20%
Functionally imp 4%
Cleft anterior mitral valve leaflet
anomalous papillary muscles and chordae
Straddling
redundant tissue tags
Juxtaposition of atrial appendages
Both appendages or left + part of right are adjacent
2-6%
Left > right -6x
Female preponderance
often additionally associated with major cardiac
pathology, including dextrocardia, VSD, bilateral
infundibulum, right ventricular hypoplasia and tricuspid
stenosis or atresia.
HAEMODYNAMICS
Fetal and Post natal physiology
Fetal circulation
Fetal circulation in TGA
TGA with VSD
in Fetus
TGA +VSD+PS
IN FETUS
POSTNATAL PHYSIOLOGY OF TGA
Determinants of effective gas exchange
Effective ventilation
Effective Pulmonary circulation
Pulmonary blood flow
Pulmonary vascular resistance
Existence of a communication between pulmonary and
systemic circuits
Persistent fetal channel – PFO or DA
Abnormal channels – ASD, VSD
Effective delivery of oxygenated blood to the tissues
Definition of shunts
Anatomical shunts
Left to Right: Blood flowing from left sided chambers
to the right sided chambers
Right to Left: Blood flowing from right sided
chambers to the left sided chambers
Definition of shunts
Physiological shunts
Left to right: The volume of oxygenated pulmonary
venous return recirculated to pulmonary circulation
(Qp – Qep)
Right to left shunt: The volume of systemic venous
return that contributes to cardiac output (reentering
the systemic circulation) without having passed
through the pulmonary circulation (Qs – Qep)
Definition of shunts
Effective pulmonary blood flow (Qep):
The volume of systemic venous return that is
effectively oxygenated in the lungs
Effective systemic blood flow (Qes):
The volume of oxygenated pulmonary venous
return that enters the systemic circulation and
perfuses the systemic capillary bed
Systemic
venous
Pulmonary
venous
return
return
Anat
R-L
RIGHT
Anat
L-R
LEFT
HEART
HEART
Physio
R-L
Physio
L-R
BODY
LUNGS
Right to Left Shunt
Systole
Left to Right Shunt
Diastole
TGA: Atrial and Ventricular level shunts
From LA to RA / LV to RV
Anatomically left to right
Physiologically, this
volume of oxygenated
blood enters systemic
circulation. Hence,
they contribute to Qes
TGA: Atrial and Ventricular level shunts
From RA to LA/ RV to LV
Anatomically, right
to left shunt
Physiologically,
this volume of
systemic venous
blood enters
pulmonary
circulation. Hence
they contribute to
Qep
TGA: Shunt at PDA level
Aorta to PA flow:
Anatomically it is left to right
Here the deoxygenated systemic venous blood enters
pulmonary circulation. Hence, this volume contributes to
Qep
PA to Aorta flow:
Anatomically it is right to left
Here the oxygenated blood enters systemic circulation.
Hence, this volume contributes to Qes
Thus, the flow across the ductus is functionally opposite to
that of flow across ASD or VSD in TGA
Initially, bidirectional flow across the ductus
Later, once the PVR falls, the flow essentially becomes
aorta to PA
The pulmonary circulation becomes overloaded fast,
especially if the PFO is restrictive
Unique feature
Net inter-circulatory mixing volume is constant: net
R-L, L-R, Qep and Qes are equal to each other
Any major difference in the volumes would result
in depletion of blood volume of one circulation at
the expense of overloading the other circulation
Precise factors controlling intercirculatory
exchange
SPECULATIVE, MULTIPLE
LOCAL PRESSURE GRADIENTS
○ Compliance of the cardiac chambers
○ Phase of respiratory cycle
○ Vascular resistances
○ Heart rate
○ Volume of blood flow
Flow across the communications
“Rules of the Heart”
With only ASD, the flow has to be bidirectional
If the flow is only or predominantly left to right across the
ASD, it suggests presence of additional shunt (VSD or PDA)
Unrestrictive VSD - flow is bidirectional
Except in the initial few days, PDA flow is always left to right
(Ao to PA).
Presence of right to left flow across ductus may suggest the
presence of coarctation of aorta
Factors influencing systemic saturation
Extent of inter-circulatory mixing and Total
pulmonary blood flow
High PBF results in increased oxygenated blood
available in the left sided chambers for mixing:
higher systemic SO2 if there is good mixing
Reduced PBF will result in low systemic SO2 in
spite of adequate anatomic shunts
Factors influencing systemic saturation
If there is delay in the fall of PVR (PPHN),
hypoxemia will persist despite adequate ASD
Need ECMO or urgent ASO
Hypoxemia provokes a fall in SVR and increase
the recirculating systemic volume
Fall in SVR may deplete the pulmonary circulation
further
Role of bronchopulmonary collaterals
Systemic arterial hypoxemia may stimulate
development of bronchpulmonary collaterals
Usually in TGA with solely a restrictive inter-atrial
communication
Prolonged survival of such infants may be due to
this extra-cardiac site of shunting/mixing
History
M:F – 4:1;unless juxtaposition of atrial
appendages
Usually in multigravida-2X increase in > 3
pregnancies
Familial recurrence-monogenic inheritance
CLINICAL MANIFESTATIONS
TGA PHYSIOLOGIC CLINICAL
CLASSIFICATION
1. TGA (IVS OR SMALL VSD) with increased PBF
and small ICS
2. TGA (VSD large) with increased PBF and large
ICS
3. TGA(VSD and LVOTO), with restricted PBF
4. TGA(VSD and PVOD),with restricted PBF
Cyanosis
As early as day 1 in pts with IVS(1st hr-56%;1st day90%)
More intense if associated PS/atresia
Mild if associated non restrictive VSD
PS often responsible for hypercyanotic spells-intense
cyanosis, tachypnea, extreme irritability and
hypothermia
Squatting is rare
Reverse differrential cyanosis
CHF
In patients with a large PDA
Large VSD – CHF develops within 1-3 wks
Mortality
1st week-30%
1st month-50%
1st year-90%
Depends on the degree of shunting
Moderate PS improves survival
Predilection for brain abscess but rare < 2 years
Arterial Pulse
Bounding pulse
- due to large volume of highly unsaturated
blood
- Not due to PDA-since only systolic shunt
from aorta to PA
Diminished femoral pulses
- CoA
- Subaortic stenosis-anterior and rightward
displacement of septum
Palpation
Normal in neonates
RV impulse
LV impulse – non restrictive VSD with low PVR
Palpable S2 A2
Auscultation
Loud A2
LV S3-mildly cyanosed patients,increased PBF,LV
failure
RV S3-deeply cyanosed patients, increased
systemic flow, RV failure
Auscultation
Ejection click-pulmonary;does not decrease with
inspiration
Aortic-subaortic stenosisdilated aortic root
MSM-aortic:hypervolemic and hyperkinetic circulation
Pulmonary: valvular- after few weeks of birth,
progressively increases
Subvalvular dynamic obstruction-3rd LICS and radiates
to the right
Auscultation
VSD: holosystolicshortensabolished
PDA:
Systolic if large PDA since high PVR curtails
diastolic flow
Continuous if restrictive PDA
MDM may be heard across AV valves
ECG
Normal in first few days of life
RAE-increased pressure(CHF) or volume (hypervolemic
systemic circulation)
LAE-large ASD,increased PBF
ECG
RVH - NR VSD + high PVR/PS
BVH - NR VSD + low PVR
Right precordial T waves not inverted but rather
distinctly taller than the left sided T waves
CXR
Absent thymic shadow after 12 hours of life
Narrow vascular pedicle - AP orientation of great vessels
Right aoric arch -11-16%
Egg on side appearance
Juxtaposition-localised bulge along the mid left cardiac
border which represents contiguous mass of the 2
appendages together
PBF & Heart size inversely proportional
D-TGA with VSD
ECHO
Diagnosis
Detection of shunt
Detection of outflow obstructions
Associated anomalies
Coronary Anatomy
Parasternal long axis view showing the 2 great vessels parallel to each
other. In a normal heart, the 2 great vessels should not appear together in
any plane as they cross each other in their proximal course. The aorta here
is anterior, not posterior as it normally should be.
Parasternal short axis view showing the aortic valve anterior to the
pulmonary valve. .
Parasternal long axis with a tilt of the probe to show the entire length of the
abnormally situated anterior aorta. The posteriorly situated pulmonary
artery can be seen to bifurcate, a clue that the posterior vessel is not an
aorta.
Subcostal view showing the pulmonary artery coming from the left ventricle
and bifurcating as it travels distally.
Cardiac catheterization in TGA
Fallacies in application of Fick’s Principle in
calculating shunts and flows in TGA
Oxygen consumption is not normal, so assumed values
are unreliable
Arteriovenous oxygen differences may be very small, so
magnitudes of errors in calculated values would be very
large.
Effect / contribution of Bronchopulmonary collaterals to
PBF – can result in overestimation.
TGA WITH NO ASSOCIATED
DEFECTS IN NEWBORN
TGA WITH LARGE VSD IN
NEWBORN
Management
Medical
Prostaglandin E1 infusion should be started to
improve arterial oxygen saturation by reopening
the ductus. This should be continued throughout
the cardiac catheterization and until the time of
surgery.
Oxygen should be administered for severe
hypoxia. Oxygen may help lower pulmonary
vascular resistance and increase PBF, resulting in
increased systemic arterial oxygen saturation.
Role of PGE1 in TGA
Considerable benefit in first few days till PVR is
elevated, especially if PFO is small
Enables bidirectional shunting, improves mixing
If valve of FO is competent, it would result in
increased LA pressure and pulmonary edema
Atrial Septostomy
Before surgery, cardiac catheterization and a
balloon atrial septostomy (i.e., the Rashkind
procedure) are often carried out to have some
flexibility in planning surgery.
a balloon-tipped catheter is advanced into the left
atrium (LA) through the PFO. The balloon is
inflated with diluted radiopaque dye and abruptly
with-drawn to the right atrium (RA) under
fluoroscopic or echo monitoring.
Atrial Septostomy
For older infants and those for whom the initial
balloon atrial septostomy was only temporarily
successful, blade atrial septostomy may be
performed.
Following this, the balloon procedure can be
repeated for a better result.
Definitive Repair
At three levels:
the atrial level : Senning or Mustard Sx
ventricular level : Rastelli operation
great artery level : arterial switch operation or Jatene
operation
Atrial level Surgery
Mustard operation: This oldest surgical technique redirects
the pulmonary and systemic venous return at the atrial
level by using either a pericardial or a prosthetic baffle.
Senning operation: This is a modification of the Mustard
operation. It uses the atrial septal flap and the RA free
wall to redirect the pulmonary and systemic venous
return
Complications
a.Obstruction to the pulmonary venous return
(<5% of all cases)
b.Obstruction to the systemic venous return (<5%
of all cases)
c.Residual intra-atrial baffle shunt (=20% of all
cases)
d.Tricuspid valve regurgitation (rare)
e.Absence of sinus rhythm (>50% of all cases)
and frequent supraventricular arrhythmias
f.Depressed RV (i.e., systemic ventricular)
function during exercise
g.Sudden death attributable to arrhythmias (3%
of survivors)
h.Pulmonary vascular obstructive disease
Arterial switch operation (or Jatene operation)
Pre requisite
An LV that can support the systemic circulation after surgery
The LV pressure should be near systemic levels at the time
of surgery, or the switch should be performed shortly after
birth (i.e., before 2 weeks of age).
In patients whose LV pressure is low, it can be raised by PA
banding, either with or without a shunt, for 7 to 10 days (in
cases of a rapid, two-stage switch operation) or for 5 to 9
months before undertaking the switch operation.
LV pressure >85% and LV posterior wall thickness >4.5 mm
appear to be satisfactory.
Pre-op
Coronary artery pattern amenable to transfer to
the neoaorta without distortion or kinking.
Risk is high when the left main or LAD coronary
artery passes anteriorly between the aorta and the
PA.
Pre-op
The left ventricular inflow and outflow tracts must
be free of significant structural abnormality.
The right ventricular outflow tract should be free of
significant stenosis.
Anatomic variants that may impact operative mortality
include
An intramural course of a coronary artery
A retropulmonary course of the left coronary artery
Multiple VSDs
Coexisting abnormalities of the aortic
Straddling AV valves
Longer duration of global myocardial ischemic (cross-
clamp)
prolonged circulatory arrest times
Complications
PA stenosis at the site of reconstruction - 5% to
10%
complete heart block - 5% to 10%.
Aortic regurgitation (AR)
late complication > 20% of patients especially PA banding
An important cause of AR may be unequal size of the
pulmonary cusps that leads to eccentric coaptation
Coronary artery obstruction
myocardial ischemia, infarction, and even death.
Rastelli operation
In patients with VSD and severe PS
The LV is directed to the aorta by creating an
intraventricular tunnel between the VSD and the
aortic valve.
A conduit is placed between the RV and the PA
Rastelli operation
Complications
conduit obstruction (especially in those containing
porcine heterograft valves)
complete heart block (rarely occurs).
This conduit needs to be replaced as the child
grows.
Pulmonary Artery Banding
Transposition associated with large VSD without LVOTO
To prevent
Heart failure
Pulmonary vascular disease
Present Indications
Presence of complex/multiple VSDs
Coexisting medical conditions that cause a delay in surgery
To train LV before switch in TGA/IVS
THANK U
MCQ
1.In a neonate with TGA false statement
a.Ductal closure can precipitate severe
desaturation
b.The pulmonary and systemic circulations are
arranged in series
c.It may be necessary to create an ASD
d.Immediate complete surgical repair is usually
indicated
2.Most common coronary anomaly in TGA
a.Single LAD
b.Single RCA
c.LCX from RCA
d.Intramural LAD
3.Reverse differential cyanosis
a.TGA with PDA+ high PVR
b.PDA with reversal
c.L TGA with reversal
d.TGA+VSD+PS+PDA
4.Wrong statement in TGA
a.TGA babies are predominantly males
b.Maternal diabetes may be associated
c.Birth weight are normal
d.Extracardiac anomalies are frequent
5.Surgical procedure of choice for a
neonate with complete TGA
a.Atrial switch surgery
b.Arterial switch surgery
c.Rastelli operation
d.Fontan operation
6.In TGA true statement
a.A2 loud
b.Arterial switch is the best option in the
presence of pulmonary obstruction
c.Cardiac catheterisation is the investigation of
choice
d.Egg on side in X ray always present
7.RVH in TGA at birth is defined by
a.Monomorphic R wave in V1
b.T upright after 1week in V1
c.RS ratio <1 in V6
d.Incomplete RBBB
8. Effective pulmonary blood flow(Qep)
a.0.9L/Min/m2
b.1.2L/Min/m2
c.1.5L/Min/m2
d.0.5L/Min/m2
9.Anatomical right to left shunt
a.0.9L/Min/m2
b.0.6L/Min/m2
c.1.5L/Min/m2
d.2L/Min/m2
10.Physiological right to left shunt equals
to
a.11.1L/Min/m2
b.15L/Min/m2
c.20L/Min/m2
d.7L/Min/m2