* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cardiology - What`s New in Medicine
Electrocardiography wikipedia , lookup
Coronary artery disease wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Heart failure wikipedia , lookup
Cardiac surgery wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Myocardial infarction wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
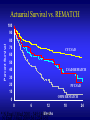
Advances in the Management of Heart Failure Daniel P. Fishbein, M.D. Professor of Medicine Medical Director, Heart Failure and Cardiac Transplantation University of Washington Medical Center The Scope of the Problem - CHF • • • • • • 5 million patients with CHF (10 million by 2020) 500,000 new cases/year 1,000,000 hospitalizations/year Incidence doubles with each decade after age 45 CHF is leading DRG $29 - 50 billion annual cost; 60% of this cost is spent on hospitalization for ADHF • CHF “epidemic” driven by aging U.S. population, improved survival of ACS, improved long-term survival • 50% of patients have preserved left ventricular ejection fraction What is Heart Failure? • Heart failure is a syndrome caused by an abnormality of cardiac function that is characterized by impaired exercised tolerance due to shortness of breath and/or fatigue, SOB at rest, systemic and pulmonary venous, and an increase in mortality due to progressive pump failure or ventricular arrhythmias • Final common pathway for a number of cardiac/cardiovascular diseases • Heart Failure with Reduced Ejection Fraction (HFrEF) • Heart Failure with Preserved Ejection Fraction (HFpEF); aka diastolic dysfunction How do we describe heart failure? • Etiology: ischemic, idiopathic, post-viral, hypertensive, toxic, valvular • NYHA Functional Class - FC I: no symptoms - FC II: symptoms with more than usual activity - FC III: symptoms with minimal activity - FC IV: symptoms at rest • ACC/AHA Stage: course of disease • Clinical assessment: – “wet” or “dry – “cold” or “warm” Clinical Assessment of Hemodynamics Cold hands Low BP Low pulse pr Tachycardia Confusion Agitation PND, Orthopnea, Edema, JVD, Rales, Effusions Heart Failure Disease Progression: ACC/AHA Heart Failure Stages D C B A Refractory End-Stage HF: Marked symptoms at rest despite maximal medical therapy Symptomatic HF: Known structural heart disease, shortness of breath and fatigue, reduced exercise tolerance Asymptomatic LVD: Previous MI, LV systolic dysfunction, asymptomatic valvular disease High Risk: Hypertension, coronary artery disease, diabetes, family history of cardiomyopathy LV=left ventricular, MI=myocardial infarction Adapted from Yancy CW et al. Prim Care Spec Ed 2002;6:15-19. Causes of Heart Failure with Reduced EF • • • • • • Coronary artery disease Ischemic cardiomyopathy Hypertensive heart disease Idiopathic cardiomyopathy Familial cardiomyopathy Valvular Cardiomyopathy – Aortic stenosis – Aortic insufficiency – Mitral regurgitation • • • • • • • • • Viral/post-viral/lymphocytic myocarditis Alcohol-related cardiomyopathy Thyroid disease (hypo or hyper) Restrictive/hypertrophic cardiomyopathy Sarcoid Cardio-toxic substances: anthracyclines, herceptin, cyclophosphamide, methamphetamines, cocaine Giant cell myocarditis Amyloid, hemochromatosis, eosinophilic myocarditis SLE, systemic sclerosis Heart Failure Symptoms • Shortness of breath and/or fatigue with activity • Shortness of breath at rest • Shortness of breath with recumbency (orthopnea) or at night (PND) – probably the most specific symptoms for heart failure • Edema • Fatigue • Chest pain • Abdominal swelling, liver pain • Poor appetite • Weight loss • Syncope • Stroke Heart Failure Signs • Rales • Evidence of pleural effusions • Elevated jugular venous pressure – the most specific physical finding for congestion • S3 – specific for LV dysfunction • Edema - when combined with JVP, specific for HF • MR murmur • Hepatomegaly • Ascites • Cool extremities • Low pulse pressure • Tachycardia CHF Initial Evaluation • Consider in patients with unexplained SOB especially with JVD, edema, evidence of pulmonary congestion, or unexplained tachycardia. • Obtain an echocardiogram: ventricular dimensions, LV and RV function, assessment of valvular regurgitation and stenosis, assessment of PA and RA pressures. • History, exam, ECG, CXR, Echo, TFTs, chemistries, BUN, creatinine, BNP, CBC with differential, UA, transferrin saturation and ferritin, consider plasma light chains, SPEP and UPEP especially in patients without ventricular dilation • Evaluation for CAD – – – – – Coronary angiography SPECT Stress PET Dobutamine stress echo CT angiography Diagnostic and Therapeutic Goals in Patients Presenting with Heart Failure • Identify underlying cause(s) of heart failure especially those that may be reversible/treatable : CAD/ischemia, valvular disease, uncontrolled HTN, thyroid abnormalities, alcohol, drugs, iron overload. • Identify conditions that may worsen heart failure: atrial arrhythmia, infection, sleep apnea, urinary obstruction, dietary and medication non-adherence, thyroid abnormalities, alcohol or drug use, meds (NSAIDS, COX 2, TZDs, CCBs, BBs). • Improve symptoms • Prevent/reverse ventricular remodeling • Prevent arrhythmic death (SCD) • Secondary prevention of AMI • Prevent stroke • Improve survival Heart Failure Pathophysiology Fall in LV Performance Cardiac output Myocardial Injury Activation of RAAS and SNS ( ET1, AVP, cytokines) Peripheral Vasoconstriction Na/Water Retention Myocardial Toxicity Gene Expression Morbidity and Mortality Remodeling and Progressive Worsening of LV Function Heart Failure Symptoms Effect of ACEI in Patients with CHF CONSENSUS and SOLVD CONSENSUS* SOLVD Treatment† NYHA Class IV NYHA Class II-III Mortality (%) 80 Placebo (n=126) 60 Placebo (n=1284) Enalapril (n=126) 40 Enalapril (n=1285) 20 0 0 6 12 18 24 Months 30 36 42 48 *Risk reduction 40% (P=0.003). †Risk reduction 16% (P=0.0036). Reprinted with permission from CONSENSUS Trial Study Group. N Engl J Med. 1987;316:1429-1435; SOLVD Investigators. N Engl J Med. 1991;325:293-302. ACEI in Patients with Systolic Dysfunction Garg et al. JAMA 1995;273:1450-6 • > 32 randomized trials of ACEI including enalapril, captopril, ramipril, quinapril and lisinopril • 23% reduction in all-cause mortality largely due to reduction in death from progressive heart failure (HR 0.69) • 35% reduction in mortality or HF hospitalization • Similar effects were observed among different ACEI • Benefit was seen across various subgroups but greatest in patients with the lowest ejection fraction High vs Low Dose Lisinopril: ATLAS Packer et al. Circulation 1999;100: 2312 - 8 • 3164 patients with FC II – IV heart failure and LVEF 30% • Low (2.5-5 mg qd) vs high (32.5 vs 35 mg qd) dose lisinopril • Median follow-up 45.7 months • Hazard Ratios: All-cause mortality 0.92 P = 0.128 CV mortality 0.90 P = 0.073 Mortality and Hosp 0.88 P = 0.002 Mortality and CHF Hosp 0.85 P < 0.001 • ~30% of patients stopped drug and 20% started on open label during course of study ARBs versus placebo in patients with chronic HF Lee V C et al. Ann Intern Med 2004;141:693-704 ARBs versus ACEI in patients with chronic HF Lee V C et al. Ann Intern Med 2004;141:693-704 ARB and ACEI combinations versus ACE inhibitors in patients with chronic HF Lee V C et al. Ann Intern Med 2004;141:693-704 ACEI and ARB in Chronic Heart Failure • Cornerstone of heart failure therapy • ACEI should be use first – improve survival, decrease hospitalization, inhibit remodeling, improve symptoms, improve functional class, inexpensive, well tolerated • Benefits of ACEI appear to be class-specific • ACEI should be uptitrated every 2-3 weeks to target dose (enalapril 10 mg bid or equivalent) • ARBs should be used in patients intolerant to ACEI (cough, angioedema) (losartan 50 mg bid or equivalent) • The benefits of ARBS are nearly equivalent to ACEI • The benefit of adding and ARB to background ACEI and -blockade has not been clearly established. Aldosterone Blockade: Rationale • Aldosterone levels associated with mortality in HF • Aldosterone is produced in tissues other than the kidney including heart and blood vessels • Aldosterone production is not completely inhibited by ACEI or ARB • Aldosterone has multiple non-renal effects including SNS activation, parasympathetic inhibition, myocardial and vascular fibrosis, baroreceptor dysfunction, vascular injury and decreased arterial compliance, reactive oxygen species, alterations in ion channels, prolonged ventricular repolarization Spironolactone 25 mg qd in patients with advanced heart failure – The Rales Trial HR= 0.70 HR SCD = 0.71 HR CV H = 0.70 Pitt, B. et al. N Engl J Med 1999;341:709-717 Eplerenone 25 – 50 mg qd in Patients with Recent AMI, EF 40% and CHF or Diabetes – The EPHESUS Trial 22 20 18 16 14 Placebo Eplerenone Cumulative 12 Incidence (%) 10 8 Total Mortality RR = 0.85; P = 0.008 6 SCD RR = 0.79; P = 0.03 4 2 0 0 3 6 9 12 15 18 21 24 27 30 33 36 Months Since Randomization EMPHASIS-HF: Eplerenone in Mild HF and LVEF ≤ 35% Zannad F et al. N Engl J Med 2011;364:11-21. Aldosterone Blockade in Chronic Heart Failure • Improve survival, decrease SCD, decrease HF hospitalization, improve symptoms and decrease ventricular remodeling in patients with FC III-IV symptoms or HF complicating recent MI • Probably a class effect, fewer side effects with eplerenone • Indicated in patients with NYHA FC 2-4 HF symptoms and reduced LVEF who can be carefully monitored for preserved renal function and normal potassium concentration • Creatinine ≤ 2.5 mg/dL in men and 2.0 mg/dL in women; potassium < 5.0 mEq/L • In EPHESUS and RALES, potassium measured at 48 hours, one, four and five weeks and every three months – study drug decreased or held for K > 5.5 mmol/L Rates of Hyperkalemia and Death After Publication of RALES 500% in spironolactone Rx (p<0.001) 275% in hosp for hyperkalemia (p<0.001) 285% in death due to hyperkalemia (p<0.001) (Juurlink, et al. NEJM 2004;351:543) Digoxin • Mild positive inotrope • Autonomic effects, sympathoinhibitory, plasma NE, plasma renin levels • Improves exercise tolerance and sxs in CHF • DIG Trial - ~7000 pts with mild – moderate CHF randomized to dig or placebo. No difference in mortality but fewer hospitalizations in pts with more severe HF • Increased mortality with higher dig levels • Use as adjunctive therapy in patients with persistent FC III-IV sxs with target dig levels < 0.8 – 1.0 ng/dL DIG Trial - Mortality N Engl J Med 1997; 336:525-33 DIG Trial –HF Hospitalization N Engl J Med 1997; 336:525-33 The DIG Trial: Survival by Gender HR = 1.23 Rathore, S. S. et al. N Engl J Med 2002;347:1403-1411 The DIG Trial: Event Rates Adjusted for Digoxin Levels (Dose Matters!) Mortality Mortality and Hospitalization Adams et al. JACC 2005; 46: 497 - 504 BETA BLOCKER TRIALS IN CHF Drug Patients Mortality (%) USCHFSG Carvedilol 1,094 65 CIBIS-II Bisoprolol 2,647 32 MERIT-HF Metroprolol XL 3,991 34 Copernicus Carvedilol 2,289 35 BEST Bucindolol 2,708 8.5 CIBIS II HR =0.68 Lancet 1999; 353:9-13 MERIT-HF Placebo Metoprolol CR/XL Cumulative mortality (%) HR = 0.65 HR SCD = 0.60 Follow-up (months) Lancet 1999; 353:2001- 07 COPERNICUS HR = 0.65 HR SCD = 0.67 Packer, M. et al. N Engl J Med 2001;344:1651-1658 Not all -blockers Work BEST N Engl J Med 2001; 344: 1659-67 Xamoterol Study Group Lancet 1990;336:1-6 Carvedilol vs Metoprolol: LV Function Metra M et al. Circulation. 2000;102:546–551. LVEF LV EDV 0 * 14 -5 *** 12 -10 -15 10 *** mL/m2 Absolute Change From Baseline LVEF units (%) 16 8 LV ESV -20 6 -25 4 -30 2 -35 ** *** *** *** -40 0 Metoprolol† (n=61) Carvedilol (n=61) 150 HF patients on diuretics, ACE inhibitors, +/- digoxin were randomized to double-blind treatment; 122 had EF/hemodynamic assessments at baseline and after 13–15 months of treatment. Achieved doses Metoprolol 124 mg/d vs Carvedilol 49 mg/day *P<.05; **P<.01; ***P<.001. †Metoprolol tartrate. -Blockers – Time Course of Improvement 45 40 LVEF % 35 30 25 20 15 10 5 0 0 1 day 1 month 3 months Hall et al JACC 1995; 25:1154-61 18 months -Blocker Dose: MOCHA Bristow, M. R. et al. Circulation 1996;94:2807-2816 Change in LVEF 6 Month Mortality Primary endpoint of mortality 40 Metoprolol tartrate Mortality (%) 30 (85 mg qd) 20 Carvedilol (42 mg qd) hazard ratio 0.83, 95% CI 0.74-0.93, P = 0.0017 10 0 0 Number at risk Carvedilol 1511 Metoprolol 1518 1 2 3 4 5 1002 933 383 352 Time (years) 1367 1359 1259 1234 1155 1105 -Blockers in Heart Failure • Cornerstone of chronic HF therapy • mortality by 35%, sudden and heart failure related death • Improve LVEF 8-12% • Benefits may be limited to the BB demonstrated in clinical trials to be effective – these are the BB that should be used to treat heart failure: carvedilol, metoprolol succinate, bisoprolol • Should be used in combination with ACEI or ARB • Initiate at low dose and up-titrate to target doses used clinical trials: carvedilol 25 mg bid; metoprolol succinate 150 mg daily; bisoprolol 10 mg daily Diuretics • No randomized controlled clinical trials to guide therapy • Goals: improve symptoms by relieving pulmonary and systemic venous congestion without impairing systemic perfusion or renal function and while maintaining normal electrolytes • Most patients need a loop diuretic • Furosemide, bumetanide, torsemide • Need to achieve a threshold dose that results in diuresis • If patients remain volume overloaded, increase the frequency of dosing, especially if using furosemide • In diuretic resistant patients, adding a thiazide or metolazone may be helpful but is associated with more hypokalemia African-American Heart Failure Trial • African-American patients with NYHA FC III – IV symptoms of heart failure for three months, LVEF 35%, treated with optimal medical therapy • Randomized to placebo vs fixed-dose combination of isosorbide dinitrate and hydralazine 20/37.5 mg tid increasing to 40/75 mg tid • Primary endpoint composite score composed of death, first CHF hospitalization and change in QOL • 1050 patients enrolled – Age 57, Men 59%, FC III 95%, LVEF 24%, ischemic 23%, hypertensive 38%, weight 93 kg, SBP 126 mm Hg, BB 74%, ACE/ARB 86% A-HeFT HR 0.57 NEJM 2004;351:2049 -57 Hydralazine and Isosorbide • Recommended for African Americans who remain symptomatic despite optimal medical therapy • Reasonable for patients who have persistent symptoms despite optimal medical therapy • Reasonable for patients with severe HF symptoms who are intolerant of ACEI or ARB, especially when this therapy is limited by hypotension or renal insufficiency • No trials data addressing the use of Hyd/ISDN in nonAfrican American patients with persistent symptoms or in patients with ACEI or ARB intolerance • Compliance difficult - tid dosing and side effects PARAGIGM - HF • Enalapril 10 mg bid vs valsartan 160 mg plus sacubitril 40 mg bid • Sacubitril is an neprilysin inhibitor • Neprilysin is a neutral endopeptidase that degrades several endogenous vasoactive peptides including natriuretic peptides, bradykinin, and adrenomedullin • Inhibition of neprilysin increases levels of these peptides and counters neurohormonal overactivation that contributes to vasoconstriction, sodium retention, and maladaptive remodeling PARADIGM-HF: Enalapril vs Valsartan and Neprilysin Inhibitor Sacubitril Pitt B et al. N Engl J Med 2014;370:1383-1392. Sudden Cardiac Death In CHF • • • • • • ~ 50% of deaths in patients with CHF are due to SCD SCD is the primary mode of death in patients with less severe CHF SCD risk factors: CAD, poor LV function, history of syncope, symptomatic ventricular arrhythmias Primary prevention strategies are limited by the lack of specific predictors of SCD – ambient ectopy, EP testing, signal averaged ECG are not useful screening studies. It has not yet been possible to identify those patients without a prior history of symptomatic arrhythmias who are at highest risk Conventional antiarrhythmic drugs increase risk of SCD ACEI, beta-blockers, and aldosterone receptor antagonists decrease SCD risk MADIT II Survival in Patients with Prior MI and LVEF ≤ 30% HR = 0.69 P = 0.007 Moss AJ. et al. N Engl J Med 2002; 346:877 SCD-HeFT - Mortality 0.4 Amiodarone vs Placebo ICD Therapy vs Placebo Mortality 0.3 HR 1.01 0.77 97.5% CI 0.86, 1.30 0.62, 0.96 P-Value 0.529 0.007 0.2 0.1 Amiodarone ICD Therapy 0 Placebo 0 6 12 18 24 30 36 42 Months of follow-up 48 54 60 Sudden Cardiac Death SCD-HeFT Mortality by NYHA Class: ICD vs. Placebo Heart Failure Trial Class II ICD Therapy 0.5 HR 1.16 Placebo 0.4 Mortality Class III 0.3 HR 0.54 97.5% CI 0.40, 0.74 0.2 48% 46% 97.5% CI 0.84, 1.61 32% 20% 0.1 0 0 12 24 36 48 Months of follow-up 60 0 12 24 36 48 Months of follow-up 60 ICD Therapy for Primary Prevention • Recommended in patients with non-ischemic dilated cardiomyopathy or ischemic heart disease with LVEF ≤ 35% and NYHA FC I – III symptoms • Patients should have a reasonable expectation of survival with good functional status for more than 1 year. • In patients with CAD, – Need to wait at least 40 days post-MI before implanting – Need to wait 3 months following CABG or PCI • In patients with non-ischemic cardiomyopathy, CMS requires that patients wait 3 months since onset of heart failure • Limitations: inappropriate shocks, RV pacing, post-shock worsening heart failure, lead complications Cardiac Resynchronization Therapy • ~ 30 - 40 % of patients with low EF and FC III-IV symptoms have a QRS duration of > 120 msec • QRS prolongation is a manifestation of abnormal cardiac conduction and has been used to identify patients with dyssynchronous ventricular contraction • Mechanical synchrony can be restored with atrial synchronized biventricular pacing (RV and LV lead) Cardiac Resynchronization Therapy • In patients with NYHA FC 2-4 HF, LVEF ≤ 35% and QRS ≥ 150 msec, CRT has been shown to: improve ventricular function; decrease MR; improve sxs, 6 minute walk distance, FC, and LV function; decrease neurohormonal activation; and decrease HF hospitalization • Meta-analysis (McAlister et al. JAMA2007; 297: 2502) of 14 randomized clinical trials of CRT demonstrated: – 37% decrease in hospitalization – 22% decrease in all-cause mortality Optimal Management of HFrEF • ACEI at target dose enalapril (10 mg bid or equivalent) – if intolerant, ARB at target dose (losartan 50 mg bid or equivalent) • Diuretics to relieve pulmonary and systemic venous congestion • Spironolactone or eplerenone with careful monitoring of potassium • Primary prevention ICD for LVEF < 36% • CRT-D for QRS ≥ 150 msec and LVEF≤ 35% Predictors of Outcome in Patients with Heart Failure • • • • • • • • • Ejection Fraction NYHA FC Recurrent hospitalization Low systolic blood pressure, pulse pressure, proportional pulse pressure Inability to initiate or need to withdrawal β-blockers or ACEI/ARB Elevated BUN and creatinine Right heart failure Diuretic dose Seattle Heart Failure Model The Seattle Heart Failure Model • Multivariate risk model to predict 1, 2, and 3 year survival in heart failure patients using easily obtainable clinical characteristics, therapy, and lab parameters • Derived from 1125 patients from the PRAISE Trial, prospectively validated in five additional cohorts (9942 patients) • Parameters included: Clinical: age, weight gender, NYHA FC, LVEF, systolic BP, presence of coronary disease Therapy: ACEI, ARB, -blocker, statin, allopurinol, aldosterone blocker, diuretic dose, ICD, BiV Pacer or ICD Labs: Hgb, % lymphocytes, uric acid, total cholesterol, serum sodium The Seattle Heart Failure Model http://depts.washington.edu/shfm/ Levy, W. C. et al. Circulation 2006;113:1424-1433 Potential Targets for New Drugs HFpEF - Clinical Picture • Most prevalent in older women with a history of hypertension, diabetes and/or coronary artery disease, frequently with a history of atrial fibrillation • Dyspnea at rest and with activity • Recurrent hospitalization for CHF, not uncommonly presenting with acute pulmonary edema • Labile and poorly controlled hypertension • Accentuated sodium and diuretic sensitivity • Atrial fibrillation poorly tolerated Causes of Heart Failure with Normal EF • Myocardial Disease Hypertension-associated hypertrophy Age Ischemic heart disease (ischemia/scarring) Diabetes/metabolic syndrome Hypertrophic cardiomyopathy Restrictive cardiomyopathy Infiltrative cardiomyopathies (amyloid, hemochromatosis,sarcoid) • Valvular Heart Disease Acute aortic insufficiency Aortic stenosis Mitral stenosis Acute mitral regurgitation • Pericardial disease Constriction, tamponade • High output states Anemia, hyperthyroidism, Paget disease, AV fistulae LV diastolic pressure-volume data Aurigemma, G. P. et al. Circulation 2006;113:296-304 HFpEF - Outcomes • Diastolic dysfunction without heart failure associated with increased risk; community based study demonstrated hazard ratios for all-cause mortality of 8.31 and 10.17 for mild and moderate-severe diastolic dysfunction (Redfield et al. JAMA 2003;289:194-202) • Hospitalization: 18% at 3.5 years (CHARM); in some studies, readmission rates may be as high as 50% • One year mortality 5 – 8% in HFpEF vs. 10 – 15% in HFrEF • Risk factors: advanced age, NYHA FC IV symptoms, CAD, decreased GFR HFpEF: Treatment • There are few clinical trials available to guide the management of patients with DHF - in fact, there is no clear evidence that patients with primary diastolic heart failure benefit from any specific drug regimen • A primary goal of therapy is control of symptoms by reducing cardiac filling pressures at rest and with activity without reducing cardiac output. • In the absence of data from controlled clinical trials, the management of DHF should be based on the control of factors known to have an important effect on ventricular relaxation/filling pressures – blood pressure, heart rate, atrial rhythm, intravascular volume, and myocardial ischemia • Underlying or exacerbating conditions should be treated – hypertension, CAD, aortic stenosis, diabetes, anemia, obesity, sleep apnea, pulmonary disease Hypertension • Reducing blood pressure improves myocardial relaxation, lowers end-systolic and diastolic volume, reduces ischemia, and results in regression of LV hypertrophy • Target BP: < 130 mmHg systolic; < 80 mmHg diastolic • ACEI/ARB: BP, improve LV relaxation, longterm may improve in hypertrophy and fibrosis • Beta-blockers/CCBs: BP, reduce ischemia, HR • Aldosterone receptor blockers: BP and may reduce hypertrophy and fibrosis HFpEF: Treatment • Diuretics should be used to relieve pulmonary congestion – patients with DHF may be at greater risk of hypotension given preload dependant stroke volume and the steep slope of the diastolic filling curve • Nitrates: lower diastolic filling pressures, may improve LV compliance and reduce ischemia • Coronary revascularization should be considered in patients with angina or significant ischemia • Atrial fibrillation: rate control; potential benefit of restoration of sinus rhythm • Reduction of HR below 60 – 80 bpm is not indicated; diastolic filling period is prolonged but ventricular filling may not be increased Lowering SBP in DHF Little and Brucks. Prog Cardiovasc Dis 2005; 47:380 - 8 TOPCAT: composite of death from cardiovascular causes, aborted cardiac arrest, or hospitalization for heart failure in patients with HF and a LVEF ≥ 45% randomized to spironolactone vs placebo Pitt et al. N Engl J Med.2014 370:1383-92 HFpEF- Conclusions • HFpEF is a common cause of heart failure, especially in older women and patients with chronic hypertension • Underlying pathophysiology is multifactorial but hypertension, aging and LV hypertrophy play important roles • Morbidity and mortality are similar in patients with systolic and diastolic heart failure • Little data from controlled clinical trials • Treatment includes control of hypertension, diuretics, management of ischemia, management of atrial fibrillation Stage D Heart Failure • Truly refractory heart failure despite optimal medical therapy • 1 year survival < 50% with optimal medical therapy • ~ 75, 000 – 200,000 patients in the U.S Stage D Heart Failure • LVEF < 25-30% • Symptoms dyspnea and/or fatigue at rest or with minimal exertion • Inability to perform most activities of daily living • Repeat/prolonged hospitalizations for ADHF • Cardiac cachexia • Diuretic resistance, refractory volume overload • Progressive end-organ dysfunction – most commonly renal dysfunction • Withdrawal of ACEI/ARB or beta blockers for hypotension or renal dysfunction • Persistent symptoms despite optimal medical therapy What do patients with Stage D heart failure look like? • “Cold and Wet” • Come to clinic in a wheel chair • Frail • Cachectic • Tachycardic • SBP < 90 mmHg • Cold hands • Pulmonary rales (but not always); elevated JVP • Abdominal distention • Lower extremity edema • Elevated BUN and creatinine • Elevated transaminases Stage D Heart Failure: Therapeutic Options • • • • Heart transplant Inotropic support Mechanical Circulatory Support Palliative Care/Hospice Heart Transplant • Heart transplant – – – – Remains the “gold standard” 1 year survival 90% and 10 year survival > 50% limited number of organs available: 2,300 U.S. Many patients with refractory heart failure are not candidates for transplant because of advanced age or comorbitity Not contraindications to Heart Transplant • Age 65 -70 years • Renal insufficiency – bridge to improvement – combined heart-kidney transplant • • • • • Prior malignancy Diabetes Hepatitis without cirrhosis HIV infection Sarcoid or amyloid-related cardiomyopathy Inotropic Support • Inotropic support – – – – – Improves symptoms and end-organ function Ambulatory setting Atrial and ventricular arrhythmias No improvement in survival Poor intermediate term outcomes Stage D HF: Inotropic Support REMATCH Medical Therapy n = 61 N Engl J Med 2001; 345:1435-43 OHSU Outpatient n = 36 J Cardiac Failure 2003; 180 – 7 Mechanical Circulatory Support • Near totally implantable devices for long-term support in patients with Stage D HF • Used as “Bridge To Transplant (BTT)” or “Destination Therapy (DT)” • Small, continuous flow, non-pulsatile devices • Durable, can function for years. • Improved survival as BTT and DT in randomized trials in Stage D heart failure • Complications: right heart failure, GI bleeding, device infection, stroke, pump thrombosis HeartMate II • Axial flow pump (nonpulsatile) • Requires anticoagulation • Can produce up to 10L/min • FDA approved HeartWare HVAD • Miniature Implantable LVAD • Intrapericardial • Magnetic/Hydrodynamic impellar suspension • 10 liters per minute • In Clinical Trials Improving Survival in LVAD Trials 100 90 HM II BTT Starling HFSA 2009 Percent Survival 80 HM II BTT Pagani JACC 2009 70 HM II BTT Miller NEJM 2007 60 HM II DT Slaughter NEJM 2009 50 40 VE DT LVAD REMATCH Rose NEJM 2001 30 XVE DT LVAD Slaughter NEJM 2009 20 Novacor DT LVAD INTrEPID Rogers JACC 2007 10 OMM REMATCH Rose NEJM 2001 OMM INTrEPID Rogers JACC 2007 0 0 6 12 Months 18 24 Triggers For Referral for Advanced Heart Failure Therapies • Two or more HF hospitalizations in the last 6 months • High diuretic dose: > 160 mg of furosemide daily • Poor renal function: BUN > 40 mg/dL; creatinine > 1.8 mg/dL • Systolic BP < 90 mmHg • Need for inotropic support • Inability to initiate ACEI or -blockers • Need to stop or decrease ACE inhibitors or -blockers • Very low LVEF – especially in younger patients • SHFM Mortality >15% ATLAS: Kaplan-Meier Analysis for Death Packer, M. et al. Circulation 1999;100:2312-2318 HeartMate II BTT Trials N Engl J Med 2007;357:885-96. • • • 133 patients at 26 centers All patients listed for transplantation (BTT) Principal outcome achieved on 100 patients (transplanted, on waiting list, recovered, or selfwithdrawn from txp list) J Am Coll Cardiol 2009;54:312–21 • • • 281 patients at 28 J Am Coll Cardiol 2011;57:1890–8 • First 169 patients centers implanted following All patients listed for FDA approval for BTT transplantation (BTT) in 2008 18 month follow-up • 77 centers (not all trial centers) data examined • Compared to concurrent INTERMACS patients • 1 year follow-up HeartMate II Results HeartMate II DT Trial • 200 patients in 38 centers • 2:1 randomization between HM2 and HM XVE (134 HM2, 66 XVE) • NYHA Class IV patients ineligible for transplantation • Primary end-point was 2 year survival free of disabling CVA or requiring device replacement HeartMate II DT Trial • 200 patients in 38 centers • 2:1 randomization between HM2 and HM XVE (134 HM2, 66 XVE) • NYHA Class IV patients ineligible for transplantation • Primary end-point was 2 year survival free of disabling CVA or requiring device replacement Actuarial Survival 100 90 Log-rank Test p=0.008 Percent Survival 80 70 60 CF LVAD 50 40 PF LVAD 30 20 10 0 0 6 12 Months 18 24 Actuarial Survival vs. REMATCH 100 90 Percent Survival 80 70 CF LVAD 60 50 40 LVAD REMATCH 30 20 PF LVAD 10 OMM REMATCH 0 0 6 12 Months * N Engl J Med 2001; 345:1435-43 18 24 Actuarial Survival vs. REMATCH 100 90 Percent Survival 80 70 CF LVAD 60 50 40 LVAD REMATCH 30 20 PF LVAD 10 OMM REMATCH 0 0 6 12 Months * N Engl J Med 2001; 345:1435-43 18 24 Results of HM2 DT Trial • Greater than 2 fold improvement in survival at 2 years compared to the pulsatile flow LVAD • Continuous flow LVADs were associated with a reduction in clinically meaningful adverse event rates • Continuous flow LVAD patients experienced early and sustained improvement in exercise capacity, functional class and quality of life Patient Selection FOR MCS: Goals Being alive and out of the hospital without a stroke or concern of a TIA while remaining free of infection and bleeding and able to ambulate unlimited distances without symptoms of heart failure enjoying the carefree life of a high school senior and able to think clearly about big thoughts remaining untethered from the VAD indefinitely and awaiting myocardial recovery while saving the healthcare system money. Ideal Candidate? • • • • • • • • Sick but not too sick Not too much right heart failure Not too much renal dysfunction Not malnourished Not too septic Not supported on mechanical ventilation for too long Not too much cerebral underperfusion Not too much noncompliance CMS Criteria for DT • • • • • • NYHA Class IV heart failure LVEF < 25% VO2max < 14 ml/kg/min Failure of OMM for 45 of last 60 days IABP dependent for 7 days Inotrope dependent for 14 days NYHA Class IV • Short of breath at rest or minimal activity • Intolerant or refractory to advanced heart failure pharmacotherapy (ACE-I, ARB, β-blocker) • Heart-failure related hospitalization in the last 6 months • • • • • • Cardiorenal syndrome Refractory Volume overload Cardiac cachexia Refractory malignant arrhythmias Inotrope dependence IABP dependence Contraindications to VAD Relative Absolute • • • • • • • • Active infection Disabling CVA Severe PVD COPD Restrictive cardiomyopathy Active substance/drug abuse with recidivism Inadequate social support • • Life expectancy < 3 years due to cause other than HF Persistent vegetative state Non-cardiac cirrhosis Not contraindications to VAD • Pulmonary HTN • Renal Insufficiency (Cr > 3.5) • Recent Substance Use/Abuse • Low Grade Malignancy (life expectancy > 5 years) • Obesity (BMI > 40) • Recent non-hemorrhagic CVA • Cardiac hepatopathy and ascites Potential Targets for New Drugs CHARM-Preserved • Average f/u: 36.6 months • Hazard ratios: CV death or CHF hospitalization - 0.89 CV death - 0.95 CHF hospitalization - 0.84 (p = 0.047) • Patients enrolled more closely resemble patients with systolic dysfunction in terms of age, gender, and etiology of heart failure CHARM-Preserved Trial • 3023 patients with Class II – IV heart failure, LVEF > 40%, and a h/o hospitalization for CV cause • Randomized to candesartan vs placebo • Target candesartan dose 32 mg qd • Primary outcome: CV death or CHF hospitalization • Clinical characteristics: Age 67.2 years Men 60% Ischemic etiology 56.4% Hypertensive etiology 22.3% h/o MI 45% DHF: Diagnostic Criteria • Clinical evidence of heart failure - Signs and symptoms of volume overload - Plasma BNP or chest x-ray - Cardiopulmonary exercise testing • Normal or mildly reduced LVEF ( 40 -50%) • Absence of valvular/pericardial disease on echo • Confirmatory evidence of diastolic dysfunction - Echo Doppler or cardiac catheterization - LV hypertrophy - Left atrial enlargement (in the absence of afib) - History of hypertension Diastolic Heart Failure: Causes • • • • • • • Hypertension-associated hypertrophy Age Ischemic heart disease (ischemia/scarring) Diabetes/metabolic syndrome Hypertrophic cardiomyopathy Restrictive cardiomyopathy Infiltrative cardiomyopathies (amyloid, hemochromatosis) • Most patients with DHF have a history of chronic hypertension that is the primary cause of their cardiac dysfunction Diastolic Heart Failure:The Scope of the Problem • 5 million patients with CHF • Incidence doubles with each decade after age 45 • 1,000,000 hospitalizations/year for acute decompensated heart failure (ADHF) • CHF is leading DRG • $29 - 50 billion annual cost • ~ 50% of patients with heart failure have normal or near normal left ventricular systolic function; these patients have been described as having “heart failure with preserved systolic function” or “diastolic heart failure (DHF)” SCD: CRT vs CRT-D CARE Companion Ellenbogen, et al. JACC 2005; 46: 2199 – 203. A-HeFT: Mechanism? NEJM 2004; 351 A-HeFT - Rationale • • • • • Prognosis worse for AA with CHF Probably less benefit from ACEI Less activation of RAAS Lower bioavailability of NO More benefit from ISDN/Hydralazine in V-HeFT trials • Hydralazine inhibits the formation of reactive oxygen species that interact with NO Heart Failure Pathophysiology • • • • • • • Myocardial injury Decreased cardiac output Neurohormonal activation – RAAS, SNS Vasoconstriction; sodium and water retention Pulmonary congestion Secondary mitral regurgitation, right heart failure Ventricular remodeling – progressive worsening in cardiac structure and function associated with abnormal myocardila gene expression and myocardial toxicity • End-organ dysfunction SFHM 1 year mortality of > 15% CRT-D in Context Ellenbogen, et al. JACC 2005; 46: 2199 – 203 HR = 0.63 CARE - HF NEJM 2005; 352: 1539 -49 HR = 0.64 CARE-HF Results The patients in the CRT Group had: • 37% reduction in mortality/hospitalization • 36% reduction in all-cause mortality • • • • Improved QOL and NHYA FC Increase in LVEF of 7% Lower ventricular volumes Less mitral regurgitation CARE-HF (NEJM 2005; 352: 1539 – 49) • CRT vs medical therapy • Patients in NSR with FC III or IV symptoms of heart failure, QRS 120 msec (with echo findings of dyssynchrony if QRS 120-149 msec), and LVEF 35% • Primary endpoint: mortality or CV hospitalization • Secondary endpoint: all-cause mortality • 813 patients at 82 centers in Europe • Age 66, Ischemic 35%, FC IV 6%, LVEF 25% • ACEI/ARB 95%, beta-blockers 72%, digoxin 43% spironolactone 57% SCD - HeFT • Placebo vs single lead ICD vs amiodarone • Patients with symptomatic heart failure (NYHA FC II – III) and LVEF 35% • Importance of optimal medical therapy emphasized • 2521 patients • Mean follow-up 45.8 months • Vital status available: 100% • 666 deaths Kaplan-Meier Event Rates Amiodarone Placebo • 1 Year • 2 Years • 2.5 Years • 3 Years • 4 Years • 5 Years 8.6% 15.4% 19.3% 24.0% 29.5% 34.0% 5.9% 14.5% 18.2% 22.4% 29.1% 36.1% ICD 6.2% 11.6% 14.2% 17.1% 22.3% 28.9% Placebo – Placebo Amiodarone – ICD -2.7% -0.9% -1.1% -1.6% -0.4% 2.1% -0.3% 2.9% 4.0% 5.3% 6.8% 7.2% MADIT II (Moss et al. NEJM 2002; 346: 877 –83) • • • • • • ICD vs conventional therapy (randomized 3:2) Prior MI ( 1 mo), LVEF 30% No requirement for EP testing or Holter screening End-point – total mortality 1232 patients Clinical characteristics: age 64 yrs, male 84%, NYHA FC I/II/III/IV 37/35/24/4%, LVEF 23 %, ACEI 70%, beta-blockers 70% • Average follow-up: 20 months • 202 deaths A-HeFT • Study prematurely stopped on recommendation of DSMB • Average follow-up 10 months • Mortality: 10.2 vs 6.2%; 54 vs 32 deaths (HR = 0.57; P=0.02) • First hospitalization: 16.4 vs 22.4% (HR = 0.67; P=0.001) • Improvement in QOL COMET • Carvedilol vs metoprolol tartrate • Patients with NYHA FC II – IV symptoms on standard treatment, LVEF < 35%, one CV hospitalization in previous two years • Stable diuretic dose > 2 weeks, ACEI > 4 weeks • Target dose: carvedilol 25 mg bid, metoprolol 50 mg bid • End-points: mortality, mortality or hospitalization • 3029 patients, Age – 62 years, LVEF 26% • Average follow-up – 58 months -Blocker Dose: MOCHA Bristow et al. Circulation 1996; 94: 2807 -16 • 345 patients with mild – moderate heart failure • Randomized to placebo vs Carvedilol 6.25mg, 12.5 mg or 25 mg po bid • Followed for six months • Primary end-point 6 minute walk test and 9 minute self-powered treadmill test • LVEF and mortality also assessed BEST NEJM 2001; 344:1659-67 ATLAS: Kaplan-Meier Analysis Showing Time to Death or Hospitalization Packer, M. et al. Circulation 1999;100:2312-2318 DIG Trial – Death or Hospitalization N Engl J Med 1997; 336:525-33 Assessment of Volume Status • Volume overload is the major cause of symptoms and hospitalization but difficult to assess in patients with chronic heart failure • JVD, rales and edema relatively specific but not sensitive physical findings in volume overloaded patients • CXR is also specific but not sensitive • Biomarkers- BNP, NT-proBNP • Thoracic impedance • Pressure sensors Volume Overload • Poor cardiac output, decrease in kidney blood flow, neurohormonal activation, thirst and secondary mitral regurgitation • left atrial and wedge pressures results in transudation of fluid into the lung that causes a decrease in lung compliance and activation of pressure receptors which result in dyspnea • Volume overload is bad because it causes symptoms and hospitalization (and may worsen angina, secondary MR, pulmonary hypertension, and remodeling) MADIT-CRT N Engl J Med 2009; 361:1329 - 38 • 1089 patients with ischemic or nonischemic cardiomyopathy, FC I – II symptoms, EF ≤ 30%, QRS ≥ 130 msec randomized 3:2 to CRT-D vs ICD • Primary end point death or heart failure event • Average fu 2.4 years • HR for primary end point 0.66 (P=0.001) • HR for nonfatal HF event 0.59 (P<0.001) • HR for all cause mortality 1.00 (P=.99) • Benefit seen only in subgroup with QRS > 150 msec MADIT-CRT N Engl J Med 2009; 361:1329 - 38 CHARM Programme 3 component trials comparing Candesartan to placebo CHARM Alternative CHARM Added CHARM Preserved n=2028 n=2548 n=3025 LVEF 40% ACE inhibitor intolerant LVEF 40% ACE inhibitor treated LVEF >40% ACE inhibitor treated/not treated Primary outcome: CV death or CHF hosp CHARM-Added: Primary outcome CV death or CHF hospitalisation 50 % Placebo 40 30 538 (42.3%) 483 (37.9%) Candesartan 20 10 Adjusted HR 0.85, p=0.010 CV Death HR 0.84, p=0.029 0 0 Number at risk Candesartan 1276 Placebo 1272 1 2 3 1176 1136 1063 1013 948 906 3.5 years 457 422 CHARM Added - Observations • • • • • • • NYHA FC 3 - 73% Mean BP 125 mmHg Enalapril dose 17 mg qd Beta-blocker use 55% Digoxin use 78% Spironolactone use 17% Not clear if ACEI plus ARB should be standard of care Aldosterone Antagonism – The Rales Trial • Spironolactone 25 mg qd vs. placebo • NYHA FC III – IV, h/o FC IV, LVEF < 35%, creatinine < 2.5 mg/dL, K < 5.0 mmol/L • 1663 patients • Age 65 years; LVEF 25%; SBP 122 mmHg; FC III/IV 70/30; Creatinine 1.2 • 30% reduction in mortality with spironolactone • Improvement in symptoms and ventricular function • Hyperkalemia 2% • Gynecomastia in 9% of men EPHESUS – Study Design • 6642 patients • Randomized 1:1 to receive eplerenone 25mg daily vs placebo increased to 50 mg daily after four weeks (mean dose 42.6 mg qd) • Potassium measured at 48 hours, one, four and five weeks and every three months – study drug decreased or held for K > 5.5 mmol/L • Primary end points: - Death from any cause - CV death or first hospitalization for CV cause • Trial designed to stop after 1012 deaths • Average follow-up: 16 months The Rales Trial • Spironolactone 25 mg qd vs. placebo • 1663 patients • Inclusion criteria - NYHA FC III – IV - h/o FC IV within previous six months - LVEF < 35%, • Exclusions: Creatinine > 2.5 mg/dL; K > 5.0 mmol/L • Spironolactone started at 25 mg qd and could be increased to 50 mg qd after 8 weeks if for worsening CHF • K checked weeks 1,4,5,8, and 12; then every 3 months Spironolactone in Patients with Advanced Heart Failure – The Rales Trial HR= 0.70 HR SCD = 0.71 HR CV H = 0.70 Pitt, B. et al. N Engl J Med 1999;341:709-717 EPHESUS: Study Design AMI, Rales (or DM ), LVEF 40%, Standard Therapy Eplerenone 25–50 mg QD n = 3100 Randomize 3–14 days Post AMI 1012 Deaths Placebo n = 3100 • All-cause mortality • CV mortality + CV hospitalization Secondary End Points: • CV mortality • CV hospitalizations • All-cause mortality + all-cause hospitalizations Other End Points: • New onset of atrial fibrillation/flutter • NYHA functional class • QOL Primary End Points: Potassium measured at 48 hours, one, four and five weeks and every three months – study .drug decreased or held for K > 5.5 mmol/L EPHESUS A. All-cause mortality C. CV death B. CV death or hospitalization D. SCD Recent Trials of CRT Do patients with normal QRS width and mechanical dyssynchrony benefit from CRT? • RethinQ (N Engl J Med 2007;357:2461-71): 172 patients with EF ≤ 35%, FC III symptoms, QRS< 130 msec and echo evidence of mechanical dyssynchrony randomized to ICD vs CRT-D. No difference in improvement in peak oxygen consumption at 6 months. Is there a better way to predict response than QRS width? • PROSPECT (Circulation 2008; 117:2608-16): Multicenter observational study to identify echocardiographic predictors of clinical response and reduction in LVESD in 498 patients who underwent CRT for standard indications. Low predictive accuracy for response for any echo measure of mechanic dyssynchrony Recent Trials of CRT Do patients with FC I – II symptoms benefit? • REVERSE (J Am Coll Cardiol 2008; 52: 1834 - 43): 262 patients with QRS ≥ 120 msec, LVEF ≤ 40%, FC I – II symptoms randomized to CRT “on’ or “off”. “on” patients had improvement in LV volumes and EF and a significant reduction in risk for first hospitalization for heart failure. No difference in survival, 6-minute walk distance of QOL. MADIT-CRT N Engl J Med 2009; 361:1329 - 38 MADIT-CRT N Engl J Med 2009; 361:1329 - 38 CRT - Caveats • Small number of FC IV patients in clinical trials (~320 patients in 9 randomized trials) • Unsuccessful implants 8 –13% • Coronary sinus injury up to 6% • Periprocedural mortality 0.4% • Lead dislodgement or malfunction 9% • Heterogeneity of response • No benefit in ~ 30% of patients • Lead placement limited by CS anatomy • Approaches to achieve optimal clinical response to CRT are in evolution Cardiac Resynchronization Therapy • ~ 30 - 40 % of patients with low EF and FC III-IV symptoms have a QRS duration of > 120 msec • QRS prolongation (delayed ventricular depolarization) identifies a HF population likely to have mechanical ventricular dyssynchrony and mechanical inefficiency • Mechanical synchrony can be restored with atrial synchronized biventricular pacing (RV and LV lead) • CRT improves ventricular function acutely in 2/3 of patients • CRT has been shown to improve sxs, 6 minute walk distance, FC, LV function, decrease neurohormonal activation, and decrease HF hospitalization in patients with moderate – severe HF and QRS prolongation What Hasn’t Worked? Vasopeptidase inhibition • OVERTURE: 5770 patients with FC II – IV symptoms randomized to enalapril vs omapatrilat (an inhibitor of both ACE and neutral endopeptidase). No difference in survival. Endothelin receptor antagonists • ENABLE I/II: low-dose bosentan (a non-selective endothelin receptor antagonist) vs placebo in patients with FC III – IV symptoms and LVEF 35%. No benefit but early worsening of heart failure early after bosentan initiation • EARTH: Darusentan four doses vs placebo in 642 patients with chronic heart failure. Well tolerated but no difference in LV ESV, symptoms, or outcome What Hasn’t Worked? TNF antagonism • RENEWAL: etanercept (TNF receptor antagonist) in 2048 patients with FC II – IV symptoms and LVEF 30%. No effect on survival or HF hospitalization. • ATTACH: two doses of infliximab (an anti-TNF monoclonal antibody) in 150 patients with FC III – IV symptoms and LVEF 35%. No effect on clinical status but increase in hospitalization in the high dose group Central sympathetic inhibition • MOXCON: moxonidine SR vs placebo in 4533 patients with FC II – IV HF. Study stopped due to an early increase in deaths and adverse events with moxonidine Other Issues • Disease management strategies: patient education, dietary counseling, compliance tools, daily weights • Treatment of sleep apnea • Treatment of anemia • Urinary retention • Physical activity and exercise training • Diuretic resistance: diuretic dose, ultrafiltration, BNP infusions • Referral for treatment of Stage D heart failure - Transplant - Destination LVAD support