* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Hydrocarbons and Functional Groups
Survey
Document related concepts
Transcript
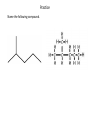
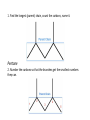
Hydrocarbons and Functional Groups Chapters 22 and 23 Organic • In Chemistry, organic means “containing carbon” • The simplest organic compounds are hydrocarbons which contain only Hydrogen and Carbon • Carbon has 4 valence electrons, and forms four covalent bonds. • It is the basis for life on Earth. Alkanes • An alkane is a hydrocarbon that contains only single covalent bonds. • Alkanes are nonpolar, do not mix with water, have weak van der Waals forces, and low boiling points. Alkenes and Alkynes • Alkenes have double covalent bonds. (ene) • Alkynes have triple covalent bonds. (yne) Memorizing Alkanes Name Molecular Formula Methane (methyl) CH4 Ethane (ethyl) C2H6 Propane (propyl) C3H8 Butane (butyl) C4H10 Pentane (pentyl) C5H12 Hexane (hexyl) C6H14 Heptane (heptyl) C7H16 Octane (octyl) C8H18 Nonane (nonyl) C9H20 Decane (decyl) C10H22 You must memorize the names of the first 10 alkanes! Naming Alkanes • 1. Find the longest (parent) chain, count the carbons, name it. • 2. Number the carbons so that the branches get the smallest numbers they can. • 3. Name the branches based on how many carbons they have, and at what carbon they branch off of. • 4. If branches appear twice, use di, 3 times use tri. • 5. Put the branch names in alphabetical order. Practice Name the following compound. 1. Find the longest (parent) chain, count the carbons, name it. Pentane 2. Number the carbons so that the branches get the smallest numbers they can. 3. Name the branches based on how many carbons they have, and at what carbon they branch off of. 2-methylpentane Practice #2 Name the following compound. 1. Find the longest (parent) chain, count the carbons, name it. Pentane 2. Number the carbons so that the branches get the smallest numbers they can. Lesson 7.0 Organic Chemistry 3. Name the branches based on how many carbons they have, and at what carbon they branch off of. 2,4-methylpentane 4. If branches appear twice, use di, 3 times use tri. 2,4-dimethylpentane Practice #3 Name the following compound. 1. Find the longest (parent) chain, count the carbons, name it. Heptane 2. Number the carbons so that the branches get the smallest numbers they can. Lesson 7.0 Organic Chemistry 3. Name the branches based on how many carbons they have, and at what carbon they branch off of. 2-methyl-3-ethylheptane* *this is NOT the final name of this molecule 5. Put the branch names in alphabetical order. 3-ethyl-2methylheptane Hydrocarbon rings • Cyclic hydrocarbons are shaped like rings instead of chains. • Benzene is a 6 carbon ring called an aromatic compound. Every other bond is a double bond. Practice • Complete the practice sheet and staple it into your notebook. • Then read Chapters 22 and 23, and answer questions 37-53 on page 719 in your notebooks. Functional Groups • A functional group is a group of atoms added to a hydrocarbon. Compound: Alcohol • The alcohol group is –OH • The functional group is called “hydroxyl” • It is used in labs, hospitals, cosmetics, fuels, and beverages Compound: Ether • An ether is a carbon chain with an Oxygen atom in it (R-O-R) the R stands for a long Carbon chain • The functional group is called “ether” • It is used in anesthesia and perfume Compound: Aldehyde • Aldehydes end in the suffix “-al” • It looks like this: • The functional group is called carbonyl • It’s used as a preservative and in flavoring Compound: Ketone • Ketones end in the suffix “-one” • They look like this: • The functional group is also called carbonyl • They are used to make plastic and in nail polish remover Compound: Carboxylic Acid • The functional group looks like this: • And it is called a carboxyl group • They are used in vinegar and in making wine Compound: Ester • An ester functional group looks like this: It’s called an ester group and is used to make fruit flavors and fragrances Compound: Amine • An amine is –NH2 attached to a long carbon chain • It’s called an amino group, and it makes up proteins and DNA. Practice • Complete the practice sheet and turn it in. • Finish reading Chapters 22 and 23, and answering questions 37-53 on page 719 in your notebooks.