* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 5 - Measurements and Calculations
Survey
Document related concepts
Transcript
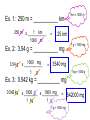
Measurement and Calculations • Chemistry – the science that deals with the materials of the universe and the changes these materials undergo • Qualitative Measurement – Qualities or observations that can be made about a substance ex: the substance is a yellow solid • Quantitative Measurement – a measurement that consists of a number and a unit ex: the substance weighs 3.45 grams Units • • tells what scale or standard is being used to represent the measurement International System (SI) • SI Base Units: – Length: meter • measures distance – Mass: grams • quantity of matter present in an sample – Volume: Liter, centimeter cubed, decimeter cubed • 1 mL = 1 cm3 1 L = 1 dm3 • three-dimensional space occupied by a sample – Temperature: Kelvin, Celsius • TK = T°C + 273 – Time: second – Pressure: Pascals – Energy/Heat: Joules – Counting Atoms: moles Units Common metric prefixes (MEMORIZE) Giga 1 x 109 _ = 1 G_ Mega 1 x 106 _ = 1 M_ Kilo 1000 _ = 1 k_ Hecto 100 _ = 1 H_ Deka 10 _ = 1 D_ (base) – meter, liter, gram… deci1 _ = 10 d_ centi1 _ = 100 c_ milli1 _ = 1000 m_ micro1 _ = 1 x 106 _ nano1 _ = 1 x 109 n_ pico1 _ = 1 x 1012 p_ * * * * * ( = lowercase Greek Mu) Scientific Notation Expresses a number as a product of a number between 1 and 10 and the appropriate power of 10 If you move the decimal pointleft positive exponent Ex: 200 g 2 1 2 x 102 g right negative exponent .00314 mL 1 2 3 3.14 x 10-3 mL Converting from Scientific Notation to Ordinary Numbers move the decimal pointpositive exponent right negative exponent left Ex: 6.32 x 101 cm 3.92 x 10-3 m 1 63.2 cm 3 2 1 .00392 m Learning Check Try these: 1. 657000000000 m 6.57 x 1011 m 2. 0.000000235 g 2.35 x 10-7 g 3. 9.34 x 102 cL 934 cL 4. 3.35 x 10-3 L 0.00335 L Limits to Measurements • When measuring you should always ______________ the _______ estimate last digit of your measurement • Your measurement should be recorded to ONE PLACE VALUE BEYOND the ______________marking calibration uncertain • Your Estimate (or _____________ number) should be the final one on the right. record the given number– • If the tool is digital, _________ no estimated number. • Measurements always have some degree of uncertainty (estimation) Ex 1: Measure the volume of liquid in the Graduated cylinder. Remember: The volume is read at the bottom of the liquid curve (called the meniscus). Ex 3: Measure the volume of liquid in the buret. Ex 2: Measure the line using both rulers. 42.8 mL 7.5 cm 7.56 cm 15.75 mL Learning check Read the following pieces of equipment, record your answer with the estimated digit and units. 1. 4. 2. 3. 5. Significant Figures All certain numbers plus first uncertain digit Rules for counting Sig. Figs. 3578 = 4 SF 1. All nonzero numbers are significant. 236 = 3 SF 2. Zeros a. Leading Zeros – precede all nonzero digits, they NEVER COUNT .0025 = 2 SF .0009 = 1 SF b. Captive (Trapped) Zeros – fall between two nonzero digits, they ALWAYS COUNT 6008 = 4 SF 20502 = 5 SF .00705 = 3 SF c. Trailing Zeros – come at the end of a number and count IF there is a DECIMAL POINT .001500300 = 7 SF 3000 = 1 SF 3000. = 4 SF 2580.0 = 5 SF 3. Exact numbers – have infinite number of sig. figs., they arise from definitions 1 inch = 2.54 cm, 1 g = 1000 mg Rounding If the digit to be removed is – a. less than 5, the preceding digit stays the same b. equal or greater than 5, increase the preceding digit by 1 When rounding off, use ONLY the first number to the right of the last significant figure Ex: Round to 3 SF $ 10,079 = $10,100 0.002978 g = 0.00298 g 0.03296 cm = 0.0330 cm 1000. mL = 1.00 x 103 mL Learning Check Determine the number of significant figures in the following numbers: a. 0.00340 g b. 9.00 mm c. 30.390 mL Round each number to 2 significant figures. a. 0.00340 g b. 9.00 mm c. 30.390 mL Calculations Notes Uncertainty in Measurement close • Accuracy: - How _________ a measurement is accepted to the actual or _________value. To evaluate know accuracy you must __________ the true value. For example, knowing a watch is 5 min not fast…The time on the watch is ________ accurate and you know it is not accurate b/c you adjustment know the real time and can make an ________. • Shooting Free Throws - Accuracy can be measured by how many are __________. baskets Precision: 1st Meaning of Precision set How close a ____________ of measurements are to the _________________. To evaluate precision you must actual value compare the values of 2 or more _______________ similar measurements. • Ex. Measure the temperature of water three times. Which set of measurements are more precise? Thermometer 1: 22.3oC, 22.3oC, 22.4oC Thermometer 2: 24.5oC, 20.1oC, 18.7oC • Shooting Free Throws - Precision can be measured by how many _______ Ex. shots in the same _________. spot side Consistently hitting the ___________ of the rim and missing. Not accurate b/c not making the shots, but precise b/c results are repeated. • Science – should be both accurate (___________) and right precise (can ____________ it consistently) repeat 2nd Meaning of Precision • Precision can also refer to how __________ a precise measurement is (more decimal __________ = places more precise) Consider mass of sugar in bubble gum – 5 g - wide range of values that it could be! - Could be between 4.5 g and 5.4 g and rounded to 5 g. – 5.0 g gives you more information – Could be between 4.95 g and 5.04 g. – 5.00 g gives you even more information – Could be between 4.995 g and 5.004 g right • More numbers to ______________ of decimal, more precise the measurement is! Learning Check Think of an example, from your life, of accuracy and precision. Multiplication and Division Number of the sig. figs. is the result of the measurement with the smallest number of sig. figs. (least accurate). LEAST NUMBER OF SIG FIGS! 3 sf 2 sf Ex 1: 4.63 m x 7.5 m 34.725 35 m2 4 sf 2 sf Ex. 2: 8.460 m2 / 2.1 m 4.0285714286 4.0 m Addition and Subtraction Align the decimal points and carry out the calculation. First column from the left with an uncertain digit determines the number of sig. figs. in your answer (Chop & round at the GAP) LEAST NUMBER of DECIMAL PLACES! Ex 1: 6.341 g + .789 g + 4.2 g 6.341 .789 4.2 GAP 11.330 11.3 g Ex. 2: 6.799 m - 2.41 m 6.799 2.41 GAP 4.389 4.39 m Learning Check 1. 22.4 L x 9.3 L 2. 9.63 g + 17.3251 g Scientific Notation and Multiplication and Division Multiplication – Multiply coefficients, ADD exponents, multiply units, round to proper S.F. Division - Divide coefficients, SUBTRACT exponents, divide units, round to proper S.F. Ex 1: (1.00 x 103 m)(3.2 x 102 m) 3.2 x 105 m2 Ex. 2: (3.00 x 104 g)/(1.0 x 102 cm3) 3.0 x 102 g/cm3 Scientific Notation and Addition and Subtraction must be in the same power of ten and same unit before you add or subtract coefficients, convert to larger exponent Ex 1: 3.0 x 1023 m + 1.0 x 1022 m 1 3.0 x 1023 m + .10 x 1023 m 3.0 GAP .10 3.10 3.1 x 1023 m Learning Check 1. 2.29 x 105 g - 9.3 x 104 g 2. 6.02 x 1023 m ÷ 1.7 x 1022 m Problem Solving and Dimensional Analysis Conversion factor – ratio of two parts of the statement that relates the two units 2.54 cm = 1 inch 100 cm = 1 m Equivalence Statement – true statement in fraction form 2.54 cm or 1 inch 1 inch 2.54 cm 100 cm or 1 m 1m 100 cm Dimensional Analysis – when used properly all units will cancel out except the desired unit Given with UNITS Wanted UNIT x ________________ # # Given UNIT Desired UNIT x ______________ = # Wanted UNIT # 1 km = 1000 m Ex. 1: 250 m = ___________ km 250 m x ___________ 1 km 1000 m = .25 km Ex. 2: 3.54 g = ___________ mg 3.54 g 1000 mg x ___________ 1 g = 1 g = 1000 mg 3540 mg 1 kg = 1000 g Ex. 3: 0.542 kg = __________ mg 0.542 kg 1000 g x __________ 1000 mg x ________ = 1 kg 1 g 1 g = 1000 mg 542000 mg Learning Check 1. 0.542 mm = __________ km 2. 0.542 g = __________ µg Determining Error accepted ___________________value - correct value based on reliable references experimental ___________________value - value measured in the lab Error = experimental value – accepted value (Note: error can be positive or absolute negative) You will take the ___________ value of this when you calculate percent error. Determining Percent (%) Error Percent error = absolute value of error divided by accepted value and multiplied by 100% % error = (experimental value – accepted value) x 100% accepted value Example: You take three temperature readings of a beaker of boiling water and record: 91.3oC, 90.9oC, and 91.1oC. Evaluate accuracy, precision, and error. Accurate? No, water boils at 100oC Precise? Yes, values are close to each other Error 1. Find average experimental data 2. Use formula (91.3oC + 90.9oC + 91.1oC)/3 = 91.1oC % error = (91.1 oC – 100 oC) 100 oC x 100% = 8.90 % Learning Check 1. At a track meet, you time a friend running 100 m in 11.00 seconds. The officials time her at 10.67 seconds. What is your percentage error? For Fun! Hagrid instructed Harry to give the delivery owl five Knuts for a newspaper (p. 62). A weekday newspaper costs $0.25. At Gringots, Harry learned that there are seventeen Sickles to a Galleon and twenty-nine Knuts to a Sickle (p. 75). Harry then paid seven Galleons for his new wand-Holly and phoenix feather (p. 85). Use dimensional analysis to calculate how much Harry’s wand would cost in dollars? 7 Galleons x ___________ 17 Sickles x ___________ 29 Knuts .25 dollars x ______________ = $172.55 1 Galleon 1 Sickles 5 Knuts On the train, Harry paid eleven Sickles and seven Knuts for junk food from the snack trolley. How much money did he spend? 29 Knuts x ___________ .25 dollars 11 Sickles x __________ = $ 15.95 5 Knuts 1 Sickle $16.30 7 Knuts .25 dollars = $ 0.35 x ___________ 5 Knuts