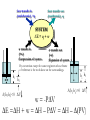
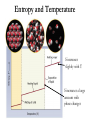
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 19
Electrolysis of water wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Stoichiometry wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Solar air conditioning wikipedia , lookup
Internal energy wikipedia , lookup
Transition state theory wikipedia , lookup
Heat transfer wikipedia , lookup
Syllabus Chemistry 102 Spring 2009 Sec. 501, 503 (MWF 9:10-10:00, 12:40-1:30) RM 100 HELD Professor: Dr. Earle G. Stone Office: Room 123E Heldenfels (HELD) Telephone: 845-3010 (no voice mail) or leave message at 845-2356 email: [email protected] (put CHEM 101-Sec. # + subject in subject line of your email) Office Hours: HELD 408: Wed. 8:00-10:50 AM I.A. Esther Ocola S.I. Leader: Analise Castellano Suggested Course Materials: “Chemistry and Chemical Reactivity, Any Edition”, by Kotz Ebook includes Online tutorial Solution manual $45 per semester Hardbound ~$160 Solution Manual ~$40 Online Tutor ~$45 Helpful 1. Dictionary of Chemistry Or online dictionary 2. Mastering the Fundamental Skills – General Chemistry I as a Second Language Useful Later As A Second Language Organic Chemistry I by Klein, There is a O-chem II and a Physics as a Second Language (Algebra based or Calculus based) for those who will have to take those classes. All College BIMS Science GEST Ag BICH, NUSC, GENE Engineering Education Geosciences Liberal Arts Agriculture other Architecture Business 493 149 118 57 35 55 18 20 10 25 0 5 100% 30% 24% 12% 7% 11% 4% 4% 2% 5% 0% 1% 501 College BIMS Science Ag BICH, NUSC, GENE GEST Engineering Education Geosciences Liberal Arts Agriculture other Architecture Business whoop RIP 2012 2011 2010 2009 2008 260 193 33 9 0 495 82 56 28 13 28 9 14 9 9 0 2 82 33% 22% 11% 5% 11% 4% 6% 4% 4% 0% 1% 33% 73% pre-something 27% widely diverging academic foci 503 242 67 62 22 31 27 9 6 5 10 0 3 100% 28% 26% 13% 9% 11% 4% 2% 2% 4% 0% 1% BIMS Science GEST Ag BICH, NUSC, GENE Engineering Education Geosciences Liberal Arts Agriculture other Architecture Business BIMS Week 1 Date 21-Jan End of Chapter Questions 6th Syllabus 9 28-Jan 2-Feb 4-Feb 6-Feb Chapter 19 1,5,29,39,49,59 11-Feb 10 Chapter 14 Sect 14.1-14.4 18-Feb 1,2,11,21,31,35, 49, 51,93 20-Feb 1,3,7,9,11,17,23, 27,41,43,47,53,55, 87,89 23-Feb 6 7 Chapter 16 4-Mar 9-Mar 11-Mar 13-Mar 8-Apr 10-Apr Reading Day 13-Apr Chapter 18 15-Apr Exam # 3 Chapters 17, 18 17-Apr 20-Apr 1,5,9,19,23,25,33, 49,63 22-Apr Chapter 20 1,3,5,13,25,31,45 27-Apr 15 6-Mar 8 1,3,9,15,19,33,35, 37,43,53,69,75,85, 99 24-Apr 27-Feb 2-Mar 13 14 25-Feb Chapter 18 6-Apr Exam #1 Chapters 14, 19 Chapter 15 61,71,93,107,109 30-Mar 3-Apr 13-Feb 5 Chapter 17 1-Apr 12 16-Feb 25-Mar Chapter 27-Mar 11 9-Feb 4 End of Chapter Questions 6th 14-Mar through 22-Mar Spring Break Date 23-Mar 30-Jan 3 Week 23-Jan 26-Jan 2 Chapter 29-Apr Exam # 4 Chapter 20 1-May Reading Day Exam #2 Chapters 15, 16 16 4-7 May Reading Days Chapter 17 17 11-May Final Sect 501 8-10 a.m. Final Sect 503 10:30 a.m.-12:30 p.m 7,11,15,23,27,35 May 11, Monday 8-10 a.m. MWF 9:10-10 a.m. May 11, Monday 10:30 a.m.-12:30 p.m. MWF 12:40-1:30 p.m. Grading: Your grade will be based on •Four one-hour examinations (each worth 200 points) •A final examination (400 points) Major Examination Schedule Spring 2009: Wed. Feb. 11 Major Exam No.1 Mon. Mar. 9 Major Exam No.2 Wed. Apr. 15 Major Exam No.3 Wed. Apr. 29 Major Exam No.4 Final Exams Section 501 Mon. May 11 8:00 to 10:00 Section 503 Mon. May 11 10:30 to 12:30 What you are used to The way the real world works +3% 80% 72% 70% 60% C B 60% 48% D,F,Q,W 90% 84% A D C 2% 16% B 100% 96% A 50% 84% 98% Percentile Rank A is > average + 1 s B is > average but less than average + 1 s C = > average - 1 s but less than average The mere formulation of a problem is far more often essential than its solution, which may be merely a matter of mathematical or experimental skill. To raise new questions, new possibilities, to regard old problems from a new angle requires creative imagination and marks real advances in science. Albert Einstein Problem - A situation that presents difficulty, uncertainty, or perplexity: Question - A request for data: inquiry, interrogation, query. Answer - A spoken or written reply, as to a question. Solution - Something worked out to explain, resolve, or provide a method for dealing with and settling a problem. 1. Numbers – Significant Figures, Rounding Rules, Accuracy, Precision, Statistical Treatment of the Data 2. Units – 5 of the 7 1. Time – seconds 2. Length – Meters Density? 3. Mass – grams Molecular Weight (Mass) 4. Amount – Moles Mole Ratio, Molarity, molality 5. Temperature – Kelvins 3. Vocabulary – Approximately 100 new terms or words and applying new or more rigid definitions to words you may already own. 4. Principles (Theories and Laws) – Stoichiometry, Quantum Theory, Bonding, Chemical Periodicity, Solutions, Thermodynamics, Intermolecular Forces, Gas Laws, Collogative Properties, Kinetics, Equilibrium, Electrochemistry cp = q/mDT DG = DH – TDS PV = nRT DT = Kmi rate = k[A]m[B]n ∆E = q + w Eocell = Ecathode = Eanode [C]c[D]d %yield = actual/theoretical * 100% K = [A]a[D]b c (ms-1) E = n = l (m) Chemistry Review The prediction of Chemical Reaction in general relies on 1. The Law of Conservation of Mass – this leads to • • Stoichiometry that allows us to compare apples and oranges Equilibrium predictions of reversible reactions which leads to • Kinetics allowing us to determine how fast the reaction will occur 2. The Law of Conservation of Energy – this leads to Thermodynamics which is stated in 3 laws 1. First Law – the energy of the Universe is constant Some Thermodynamic Terms Thermodynamics - The study of the relationship between heat, work, and other forms of energy. Thermochemistry - A branch of thermodynamics which focuses on the study of heat given off or absorbed in a chemical reaction. Temperature - An intensive property of matter; a quantitative measurement of the degree to which an object is either "hot" or "cold". 1.There are 3 scales for measuring temperature •Fahrenheit - relative •32 F is the normal freezing point temperature of water; 212 F is the normal boiling point temperature of water. •Celsius (centigrade) - relative •0 C is the normal freezing point temperature of water; 100 C is the normal boiling point temperature of water. •Kelvin - absolute •0 K is the temperature at which the volume and pressure of an ideal gas extrapolate to zero. Some Thermodynamic Terms Heat (q) - A form of energy associated with the random motion of the elementary particles in matter. Heat capacity - The amount of heat needed to raise the temperature of a defined amount of a pure substance by one degree. Specific heat - The amount of heat needed to raise the temperature of one gram of a substance by 1 C (or 1 K) •SI unit for specific heat is joules per gram-1 Kelvin-1 (J/g-K) Calorie - The specific heat of water = 4.184 J/g-K Molar heat capacity - The amount of heat required to raise the temperature of one mole of a substance by 1 C (or 1 K) •SI unit for molar heat capacity is joules per mole-1 Kelvin-1 (J/mol-K) Btu (British thermal unit) - The amount of heat needed to raise the temperature of 1 lb water by 1 F. NOTE: The specific heat of water (4.184 J/g-K) is very large relative to other substances. The oceans (which cover over 70% of the earth) act as a giant "heat sink," moderating drastic changes in temperature. Our body temperatures are also controlled by water and its high specific heat. Perspiration is a form of evaporative cooling which keeps our body temperatures from getting too high. Some Thermodynamic Terms Latent Heat versus Sensible Heat Sensible heat - Heat that can be detected by a change in the temperature of a system. Latent heat - Heat that cannot be detected because there is no change in temperature of the system. e.g. The heat that is used to melt ice or to evaporate water is latent heat. There are two forms of latent heat: •Heat of fusion - The heat that must be absorbed to melt a mole of a solid. •e.g. melting ice to liquid water •Heat of vaporization - The heat that must be absorbed to boil a mole of a liquid. •e.g. boiling liquid water to steam Some Thermodynamic Theories Caloric Theory of Heat •Served as the basis of thermodynamics. •Is now known to be obsolete •Based on the following assumptions •Heat is a fluid that flows from hot to cold substances. •Heat has a strong attraction to matter which can hold a lot of heat. •Heat is conserved. •Sensible heat causes an increase in the temperature of an object when it flows into the object. •Latent heat combines with particles in matter (causing substances to melt or boil) •Heat is weightless. The only valid part of the caloric theory is that heat is weightless. Heat is NOT a fluid, and it is NOT conserved. Some Thermodynamic Theories Kinetic Theory of Heat 1. Divides the universe into two parts: A. System. - The substances involved in the chemical and physical changes under investigation: In chemistry lab, the system is the REACTANTS inside the beaker. B. Surroundings - Everything not included in the system, i.e. the rest of the universe. 2. A BOUNDARY separates the system and the surroundings from each other and can be tangible or imaginary. A. Heat is something that is transferred back and forth across boundary between a system and its surroundings B. Heat is NOT conserved. Some Thermodynamic Theories The kinetic theory of heat is based upon the last postulate in the kinetic molecular theory which states that the average kinetic energy of a collection of gas particles is dependent only upon the temperature of the gas. where R is the ideal gas constant (0.08206 L-atm/mol-K) and T is temperature (Kelvin) The kinetic theory of heat can be summarized as follows: When heat enters a system, it causes an increase in the speed at which the particles in the system move. • The set of conditions that specify all of the properties of the system is called the thermodynamic state of a system. • For example the thermodynamic state could include: – The number of moles and identity of each substance. – The physical states of each substance. – The temperature of the system. – The pressure of the system. Standard States and Standard Enthalpy Changes 1. Thermochemical standard state conditions • The thermochemical standard T = 298.15 K. • The thermochemical standard P = 1.0000 atm. – Be careful not to confuse these values with STP. 2. Thermochemical standard states of matter • For pure substances in their liquid or solid phase the standard state is the pure liquid or solid. • For gases the standard state is the gas at 1.00 atm of pressure. • For gaseous mixtures the partial pressure must be 1.00 atm. • For aqueous solutions the standard state is 1.00 M concentration. Some Thermodynamic Terms 1. State Functions are independent of pathway: – T (temperature), P (pressure), V (volume), DE (change in energy), DH (change in enthalpy – the transfer of heat), and S (entropy) 2. Examples of non-state functions are: – n (moles), q (heat), w (work) The Three Laws of Thermodynamics There are two basic ideas of importance for thermodynamic systems. 1. Chemical systems tend toward a state of minimum potential energy. 2. Chemical systems tend toward a state of maximum disorder. The First Law of Thermodynamics • The first law is also known as the Law of Conservation of Energy. Energy is neither created nor destroyed in chemical reactions and physical changes. •The energy of the universe does not change. •The energy in a system may change, but it must be complemented by a change in the energy of its surroundings to balance the change in energy. The term internal energy is often used synonymously with the energy of a system. It is the sum of the kinetic and potential energies of the particles that form the system. The last postulate in the kinetic molecular theory states that the average kinetic energy of a collection of gas particles is dependent only upon the temperature of the gas. The First Law of Thermodynamics Esys = KEsys + PEsys 1. 2. KE – kinetic energy: translational, rotational, vibrational PE – energy stored in bonds (Bond energy) The First Law of Thermodynamics If a system is more complex than an ideal gas, then the internal energy must be measured indirectly by observing any changes in the temperature of the system. The change in the internal energy of a system is equal to the difference between the final and initial energies of the system: The equation for the first law of thermodynamics can be rearranged to show the energy of a system in terms of the energy of its surroundings. This equation indicates that the energy lost by one must equal the energy gained by the other: The First Law of Thermodynamics The energy of a system can change by the transfer of work and or heat between the system and its surroundings. Any heat that is taken, given off, or lost must be complemented by an input of work to make up for the loss of heat. Conversely, a system can be used to do any amount of work as long as there is an input of heat to make up for the work done. This equation can be used to explain the two types of heat that can be added to a system: 1. Heat can increase the temperature of a system. This is sensible heat. 2. Heat that does ONLY WORK on a system is latent heat. The First Law of Thermodynamics 1. Exchange of heat (q) Endothermic and exothermic 2. Work is performed (w) DE = q + w Solids, Liquids, Solutions Changes in volume are negligible Therefore w is effectively zero DE = q + 0 = DH DH is change in enthalpy which is the transfer of heat and is measured experimentally by determining changes in temperature. Gases Why only gases? Because changes in volume results in work w = Fd F = Pressure x Area d = Dh W = P (A Dh) = DV h heat transfer in (endothermic), +q heat transfer out (exothermic), -q SYSTEM ∆E = q + w w transfer in (+w) Compression of system w transfer out (-w) Expansion of system By convention except for some engineers whose frame of reference is the work done on the surroundings. hi hf A(hf-hi)<0 DV hi hf A(hf-hi)>0 DV w = -PDV DE =DH + w = DH – PDV = DH – D(PV) DE = DH – D(PV) Constant Volume Constant Pressure w = -PDV DV = 0 DE = DH Check the temperature change Apply some stoichiometry And the Ideal Gas Law PV=nRT D(PV)=D(nRT) Hold Temperature constant k1 D(PV)=D(nRk1) Combine constants and multiply through by -1 -D(PV) = -R1Dn w = -PDV = -R1Dn DE = DH + w = DH - R1Dn DE DH Dn DE = DH exothermic No change DE = DH endothermic No change DE > DH exothermic increase DE > DH endothermic decrease DE < DH exothermic decrease DE < DH endothermic increase Thermochemical Equations • Thermochemical equations are a balanced chemical reaction plus the DH value for the reaction. – For example, this is a thermochemical equation. C5 H12( ) 8 O 2(g) 5 CO 2(g) 6 H 2 O ( ) 3523 kJ 1 mole 8 moles 5 moles 6 moles • The stoichiometric coefficients in thermochemical equations must be interpreted as numbers of moles. • 1 mol of C5H12 reacts with 8 mol of O2 to produce 5 mol of CO2, 6 mol of H2O, and releasing 3523 kJ is referred to as one mole of reactions. Thermochemical Equations Write the thermochemical equation for CuSO4(aq) + 2NaOH(aq) 50.0mL of 0.400 M CuSO4 at 23.35 oC 50.0mL of 0.600 M NaOH at 23.35 oC Tfinal 25.23oC CH2O = 4.184 J/goC Density final solution = 1.02 g/mL Cu(OH)2(s) + Na2SO4(aq) The Second Law of Thermodynamics • The second law of thermodynamics states, “In spontaneous changes the universe tends towards a state of greater disorder.” • Spontaneous processes have two requirements: 1. The free energy change of the system must be negative. 2. The entropy of universe must increase. • Fundamentally, the system must be capable of doing useful work on surroundings for a spontaneous process to occur. Changes in DS are usually quite small compared to DE and DH. Notice that DS has units of only a fraction of a kJ while DE and DH values are much larger numbers of kJ. The Second Law of Thermodynamics Entropy (S) - A measure of the disorder in a system. Entropy is a state function. where k is a proportionality constant equal to the ideal gas constant (R) divided by Avogadro's number (6.022 x 10-23) and lnW is the natural log of W, the number of equivalent ways of describing the state of a system. In this reaction, the number of ways of describing a system is directly proportional to the entropy of the system. The Second Law of Thermodynamics Number of Equivalent Combinations for Various Types of Poker Hands Hand W ln W Royal flush (AKQJ10 in one suit) 4 1.39 36 3.58 624 6.44 3,744 8.23 Flush (five cards in the same suit) 5,108 8.54 Straight (five cards in sequence) 10,200 9.23 Three of a kind Two pairs One pair No pairs 54,912 123,552 1,098,240 1,302,540 2,598,960 10.91 11.72 13.91 14.08 Straight flush (five cards in sequence in one suit) Four of a kind Full house (three of a kind plus a pair) Total The Second Law of Thermodynamics Entropy of Reaction (DS) •The difference between the sum of the entropies of the products and the sum of the entropies of the reactants: In the above reaction, n and m are the coefficients of the products and the reactants in the balanced equation. As with DH, entropies have been measured and tabulated in Appendix L as So298. When: DS > 0 disorder increases (which favors spontaneity). DS < 0 disorder decreases (does not favor spontaneity). The Second Law of Thermodynamics Natural processes that occur in an isolated system are spontaneous when they lead to an increase in the disorder, or entropy, of the system. Isolated system - System in which neither heat nor work can be transferred between it and its surroundings. This makes it possible to ignore whether a reaction is exothermic or endothermic. If DSsys > 0, the system becomes more disordered through the course of the reaction If DSsys < 0, the system becomes less disordered (or more ordered) through the course of the reaction. The Second Law of Thermodynamics There are a few basic principles that should be remembered to help determine whether a system is increasing or decreasing in entropy. •Liquids are more disordered than solids. •WHY? - Solids have a more regular structure than liquids. •Gases are more disordered than their respective liquids. •WHY? - Gases particles are in a state of constant random motion. •Any process in which the number of particles in the system increases consequently results in an increase in disorder. • In general for a substance in its three states of matter: Sgas > Sliquid > Ssolid The Second Law of Thermodynamics Does the entropy increase or decrease for the following reactions? • • • • Answers: •INCREASES - The number of particles in the system increases, i.e. one particle decomposes into two. In addition, one of the products formed is a gas which is much more disordered than the original solid. •DECREASES - The number of particles in the system decreases, i.e. there are four moles of gas reactants and only 2 moles of gas products. •INCREASES - The number of particles in the system increases, i.e. the single reactant dissociates into two ion particles. In addition, the ions in the ionic solid are organized in a rigid lattice structure whereas the ions in aqueous solution are free to move randomly through the solvent. •DECREASES - The reactant changes from a gas to a liquid, and gases are more disordered than their respective liquids. • • Entropy, S The Third Law of Thermodynamics states, “The entropy of a pure, perfect, crystalline solid at 0 K is zero.” This law permits us to measure the absolute values of the entropy for substances. – To get the actual value of S, cool a substance to 0 K, or as close as possible, then measure the entropy increase as the substance heats from 0 to higher temperatures.The coldest place in nature is the depths of outer space. There it is 3 degrees above Absolute Zero. – Notice that Appendix L has values of S not DS. Predicted 1924......Created 1995 Bose-Einstein Condensation in a gas: a new form of matter at the coldest temperatures in the universe... A. Einstein S. Bose Cornell and Wieman cooled a small sample of atoms down to only a few billionths (0.000,000,001) of a degree above Absolute Zero Entropy, S BEC Entropy and Temperature S increases slightly with T S increases a large amount with phase changes Entropy, S • Entropy changes for reactions can be determined similarly to DH for reactions.This is only true, i.e. conserved, for the system. This is not included for the surroundings. DS o 298 = nS o products n - nS o reactants n Entropy, S • Calculate the entropy change for the following reaction at 25oC. Use Appendix L. 2 NO2(g) N 2 O 4(g) You do it! Entropy, S • Calculate DSo298 for the reaction below. Use Appendix L. 3 NO(g N 2 O (g NO2(g You do it! Free Energy Change, DG, and Spontaneity • • In the mid 1800’s J. Willard Gibbs determined the relationship of enthalpy, H, and entropy, S, that best describes the maximum useful energy obtainable in the form of work from a process at constant temperature and pressure. – The relationship also describes the spontaneity of a system. The relationship is a new state function, DG, the Gibbs Free Energy. DG = DH-TDS at constant T and P Free Energy Change, DG, and Spontaneity • • The change in the Gibbs Free Energy, DG, is a reliable indicator of spontaneity of a physical process or chemical reaction. – DG does not tell us how quickly the process occurs. • Chemical kinetics, the subject of Chapter 16, indicates the rate of a reaction. Sign conventions for DG. – DG > 0 reaction is nonspontaneous – DG = 0 system is at equilibrium – DG < 0 reaction is spontaneous Free Energy Change, DG, and Spontaneity • Changes in free energy obey the same type of relationship we have described for enthalpy, DH, and entropy, DS, changes. DG 0 298 = nDG n 0 products - nDG n 0 reactants Free Energy Change, DG, and Spontaneity • Calculate DGo298 for the reaction in Example 15-8. Use Appendix L. C3H8( ) 5 O 2(g) 3 CO2(g) 4 H 2O( ) You do it! The Temperature Dependence of Spontaneity • Free energy has the relationship DG = DH -TDS. • Because 0 ≤ DH ≥ 0 and 0 ≤ DS ≥ 0, there are four possibilities for DG. Forward reaction DH <0 <0 >0 >0 DS >0 <0 >0 <0 DG <0 T dependent T dependent >0 spontaneity at all T’s. at low T’s. at high T’s. Nonspontaneous at all T’s. Equilibrium Spontaneous Non Spontaneous Spontaneity is favored when DH < 0 DS > 0 DG = DH -TDS DG DH .. ) ) High T .. .. ) .. .. .. ) ) .. ) ) Low T .. ) .. DS ) .. ) DG = 0 DG < 0 DG > 0 The Temperature Dependence of Spontaneity • • • Calculate DSo298 for the following reaction C3H8(g) + 5 O2(g) ) 3 CO2(g) + 4 H2O(g) We know that DHo298= -2219.9 kJ, and that DGo298= -2108.5 kJ.