* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Energy
Physical organic chemistry wikipedia , lookup
Eigenstate thermalization hypothesis wikipedia , lookup
Thermodynamics wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Enzyme catalysis wikipedia , lookup
Marcus theory wikipedia , lookup
Heat transfer physics wikipedia , lookup
George S. Hammond wikipedia , lookup
History of thermodynamics wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
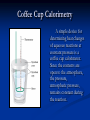
Thermochemistry The study of energy and its transformations Definitions When examining chemical systems or reactions, in order to keep track of energy changes, we consider the system and its surroundings. The system is where we put our focus. Typically, it is the reactants and products. The surroundings include everything else in the universe. Often, we just consider the immediate surroundings, such as the reaction vessel. Energy Changes Chemical reactions and physical changes typically involve a transfer of energy. If a process such as melting ice requires energy, the reverse process of freezing water releases the same amount of energy. Definitions If a reaction results in the evolution of heat, energy flows out of the system and into the surroundings. These reactions are exothermic. The energy lost by the system must be equal to the energy gained by the surroundings. Definitions If a reaction requires heat, energy flows from the surroundings into the system. These reactions are endothermic. The energy gained by the system must be equal to the energy lost by the surroundings. Physical Changes and Energy Changing the state of a substance involves an energy change. In melting or boiling a substance, the attractive forces between the atoms or molecules must be overcome, and heat is required. This process is endothermic. When a substance cools and condenses or freezes, heat is given off, and the process is exothermic. Chemical Reactions and Energy In chemical reactions, the energy changes result from the breaking and the formation of chemical bonds. Bond breaking always requires energy. Bond making always releases energy. Chemical Reactions and Energy Bond breaking always requires energy. Bond making always releases energy. In exothermic reactions, more energy is released in forming the products than is used in breaking apart the reactants. Types of Systems Systems can be open (both energy and matter are exchanged, closed (only energy is exchanged) and isolated (neither energy nor matter is exchanged with the surroundings.) Definitions - Energy Chemical systems contain both kinetic energy and potential energy. Energy is the capacity to do work or to produce heat. An example of both is the combustion of gasoline. The gaseous products expand and do work (moving the pistons of an engine) and the reaction also produces heat. Definitions - Energy Energy is the capacity to do work or to produce heat. Heat and work are ways that objects can exchange energy. Definitions - Energy Kinetic energy is the energy of motion, and it depends upon the mass of the object and its velocity. Since molecules, especially those of gases, are in motion, they posess kinetic energy. Definitions- Energy Potential energy is energy due to position or composition. Chemical energy is potential energy due to composition. For example, gasoline and oxygen have the potential to produce energy if they react. Internal Energy The internal energy (E, or U) of a system is the sum of the kinetic and potential energies of all of the particles of the system. It is generally not possible to determine the internal energy of a system, but we can measure changes in internal energy. Internal energy is changed by the flow of work and/or heat. Internal Energy Internal Energy is a state function. That is, it depends solely on the present state of the system, and not how it may have gotten to a particular state. A state function is independent of pathway. Internal Energy Internal energy (E or U) is a state function, and depends only on the state of the system, and not how it got to that state. Internal Energy & the st 1 Law The First Law of Thermodynamics states that: Energy can be converted from one form to another, but cannot be created or destroyed. It is not possible to measure the total energy of a system, but it is possible to determine changes in energy. Internal Energy Since energy may flow to or from the surroundings and the system, we are concerned with energy changes rather than the absolute value of the internal energy. ΔE = Efinal – Einitial (some texts use the symbol U for internal energy) The st 1 Law of Thermodynamics Since the energy change of the system is equal and opposite to the energy change of the surroundings, ΔEsystem= –Δesurroundings or ΔUsystem= –ΔUsurroundings The st 1 Law of Thermodynamics The implication of the first law of thermodynamics is that: The energy of the universe is constant. Work and Heat Energy is the capacity to do work or transfer heat. The internal energy of a system changes when heat is exchanged, or when the system does work on its surroundings, or when the surroundings do work on the system. ΔE = q + w or ΔU = q + w Energy, Work and Heat As the system loses energy to the surroundings, it can do so by losing heat (q), and/or doing work (w). As a result, ΔE = heat + work ΔE = q + w ΔE = q -PΔV Work Chemical reactions can be harnessed to do electrical work (as with batteries), or expansion work (such as expanding gases in an internal combustion engine). Our focus will be on expansion work. Work Expansion work results when a reaction produces more gaseous products than reactants, and thus pushes back the atmosphere as the reaction proceeds. If the volume of the system contracts during reaction (more gaseous reactants than products), work is done by the surroundings on the system. Expansion Work Usually we just consider the volumes of gases in a chemical reaction. 2 H2O(g) 2 H2(g) + O2(g) Since 2 moles of gaseous reactants produce 3 moles of gaseous products, the system expands, and does work in pushing back the atmosphere. Expansion Work work = Force x Distance Pressure = Force/Area, or Force = Pressure(Area) work = Pressure(Area) x Distance work = Pressure(Area) x ∆h work = Pressure (length x width) x ∆h work = P ∆V Expansion Work work = P ∆V If gases are produced by a reaction and the volume expands, the system is doing work on the surroundings. The sign, when considering the system, must be negative. So, work = - P ∆V Energy, Work and Heat ΔE = q –PΔV Solving for q, the heat change, q = ΔE + PΔV The heat change, q, will vary with the reaction conditions. Reactions in an open vessel are performed at constant pressure, those in a sealed vessel are performed at constant volume. Reactions at Constant Volume q = ΔE + PΔV At constant volume, expansion work isn’t possible, and the heat change, qv, equals the change in internal energy. indicates constant qv = ΔE volume or qv = ΔU Energy, Work and Heat q = ΔE + PΔV At constant pressure, q becomes qp, and qp = ΔE + PΔV or qp = ΔU + PΔV denotes constant pressure Energy, Work and Heat q = ΔE + PΔV At constant pressure, q becomes qp, and qp = ΔE + PΔV or qp = ΔU + PΔV denotes constant pressure Energy and Enthalpy qp = ΔE + PΔV Since an open vessel is such a common apparatus, the heat transferred at constant pressure is given its own name, the enthalpy change, ΔH. qp = ΔE + PΔV = ΔH Enthalpy Enthalpy is a state function, and is independent of reaction pathway. ΔH = Hfinal-Hinitial ΔH = Hproducts-Hreactants Standard Conditions Many reactions are categorized by their standard enthalpy change, ΔHo. The degree sign indicates standard conditions. Standard conditions specify that the reactants and products are in the same molar amounts represented by the coefficients in the balanced chemical reaction. Standard Conditions In a given experiment, the quantities of reactants and enthalpy change will vary, but the standard enthalpy change is reported based on molar quantities. The enthalpy of a reaction will also vary with the physical states of reactants or products as well as temperature and pressure. Standard Conditions A thermodynamic standard state refers to a specific set of conditions. The standard is used so that values of enthalpy changes can be directly compared. The standard state is the most stable form of a substance at 1 atm pressure and 25oC. Standard Conditions Standard conditions are indicated using a degree symbol ( o ). Standard conditions for thermochemical data differ from the standard conditions used in the gas laws. 1. All gases have a pressure of exactly 1 atm. 2. Pure substances are in the form that they normally exist in at 25oC and 1 atm pressure. 3. All solutions have a concentration of exactly 1M. Standard Conditions For example, since oxygen is a diatomic gas at 25oC, the standard state of oxygen is O2(g) at a pressure of 1 atm. Thermochemical Equations Chemical reactions (or changes of state) may be written with their enthalpy of reaction. For example, CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l) ΔH=–890.4 kJ/mol The enthalpy change is for the reaction written, assuming molar quantities. 890.4 kJ of heat is produced when a mole of CH4(g) is burned. Thermochemical Equations If the reaction is reversed, 890.4 kJ is required to produce each mole of CH4(g). CO2(g) + 2 H2O(l) CH4(g) + 2 O2(g) ΔH=+890.4 kJ/mol If the coefficients in a balanced reaction are multiplied by an integer, the value of ∆H is multiplied by the same integer. Calorimetry Calorimetry is the science of measuring heat. It typically involves measuring temperature changes as a substance loses or gains heat. Since substances vary in how much their temperature changes as heat is lost or gained, it is important to know the heat capacity (C) of substances involved in the reaction. Heat Capacity (C) The heat capacity of a substance is the amount of heat absorbed, usually in joules, per 1 degree (C or K) increase in temperature. The amount (mass) of the substance also determines the amount of heat lost or gained. C = heat absorbed = _q_ increase in temp. ΔT Heat Capacity The specific heat capacity is for a gram of a substance. It has the units J/oC-g or J/K-g. The molar heat capacity is for a mole of a given substance. It has the units J/oC-mol or J/K-mol. Calorimeter Constant In measuring heat changes during a reaction, any heat absorbed or lost be the calorimeter (the apparatus itself) must be considered. If this amount of heat is significant, the calorimeter constant may be provided or measured. This is the heat capacity of the specific apparatus used, and is expressed in J or kJ per degree change in temperature (K or oC). Coffee Cup Calorimetry A simple device for determining heat changes of aqueous reactions at constant pressure is a coffee cup calorimeter. Since the contents are open to the atmosphere, the pressure, atmospheric pressure, remains constant during the reaction. Coffee Cup Calorimetry The heat change for the reaction, qp, is equal to the enthalpy change for the reaction. If heat is given off, it goes towards warming up the contents of the calorimeter and toward warming up the calorimeter walls, thermometer, stirrer, etc. Coffee Cup Calorimetry qreaction = qcontents + qcal qcontents = (mass of solution) (ΔTsoln)Csoln Csoln is the specific heat capacity of the reaction mixture. If solutions are aqueous and fairly dilute, the specific heat capacity of water, 4.18J/oC-g, may be used. Coffee Cup Calorimetry qreaction = qcontents + qcal qcal = Ccal (ΔT) Ccal is the calorimeter heat capacity. It includes the heat needed to warm up the walls, thermometer and stirrer of the calorimeter, along with any heat loss due to leaks. In many simple calculations, Ccal is assumed to be negligible, and may be ignored. Obtaining ΔH of Reaction The enthalpy change of a reaction, ΔH, can be obtained from qp. First, a sign must be assigned. If the temperature increased during the reaction, the reaction is exothermic, and q is negative. If the temperature decreased during the reaction, the reaction is endothermic, and q is positive. Obtaining ΔH of Reaction The enthalpy change of a reaction, ΔH, can be obtained from qp. Also, qp is for a specific quantity of reactants. Typically, ΔHrxn is for molar quantities of reactants. To calculate ΔHrxn from qp, you must calculate the heat change per mole of reactant. Problem: Calorimetry A coffee cup calorimeter contains 125. grams of water at 24.2oC. A 10.5 g sample of KBr, also at 24.2oC, is added. After dissolving, the mixture reaches a final temperature of 21.1oC. Calculate ∆Hsoln in joules/gram and kJ/mol. Assume the specific heat of the solution is 4.18 J/g-oC, and no heat is transferred to or from the calorimeter or surroundings. Constant Volume Calorimetry Certain reactions, notably combustion reactions, do not lend themselves to open vessels. These reactions are usually carried out in a sealed reaction vessel called a bomb calorimeter. The bomb calorimeter is a rigid steel container that is sealed after the reactants have been added. The reaction takes place once an electrical current is sent through an ignition wire to the reaction mixture. Bomb Calorimetry The steel bomb is immersed in an insulated bath containing either water or mineral oil. As the combustion reaction releases heat, the heat is transferred to the bath. Bomb Calorimetry Reaction vessel Ignition wire O2 inlet gaskets Bomb Calorimetry Once the bomb has been charged with reactants, it is placed in the water or oil bath until it reaches a constant temperature. A current is sent through the ignition wire, and the combustion reaction takes place. The heat given off by the reaction is evident from the increase in temperature of the water/oil bath. Bomb Calorimetry The heat generated by the reaction warms up the contents of the calorimeter (the bomb, thermometer, container walls, stirrer) and the water (or oil) bath. Usually, the calorimeter constant, which is the heat capacity of the entire apparatus, is provided, or determined by combusting a substance with a known energy of combustion. Problem: Bomb Calorimetry The energy released by combustion of benzoic acid is 26.42 kJ/g. The combustion of .1584g of benzoic acid increases the temperature of a bomb calorimeter by 2.54 oC. a) Calculate the calorimeter constant. Problem: Bomb Calorimetry b) 0.2130 g of vanillin (C8H8O3) is burned in the same calorimeter with a temperature increase of 3.25oC. Calculate the energy of combustion of vanillin in kJ/g and kJ/mol. Hess’s Law Enthalpy is a state function. This means that a change in enthalpy depends solely on the initial and final states (products and reactants), and is independent of the reaction pathway. Hess’s Law Hess’s Law is a method that combines related chemical reactions and their enthalpy changes. Since enthalpy changes are independent of pathway, as long as the net reaction matches the reaction of interest, the sum of the enthalpy changes will yield ΔH for the desired reaction. Hess’s Law Hess’s Law There are a few basic rules in applying Hess’s Law: 1. If a reaction is reversed, the sign of ΔH is also reversed. 2. If the coefficients in a balanced reaction are multiplied by an integer, the value of ∆H is multiplied by the same integer. Problem: Hess’s Law Calculate ∆Ho for the reaction: C6H4(OH)2(aq) + H2O2(aq) C6H4O2(aq) + 2 H2O(l) Using: 1)C6H4(OH)2(aq) C6H4O2(aq) + H2(g) ∆Ho = +177.4 kJ 2) H2 (g) + O2(g) H2O2(aq) ∆Ho = –191.2 kJ 3) H2 (g) + 1/2 O2(g) H2O(g) ∆Ho = –241.8 kJ 4) H2O(g) H2O(l) ∆Ho = –43.8 kJ Standard Enthalpies of Formation A formation reaction involves combining elements, in their standard states, to form one mole of a compound. A table of standard enthalpies of formation (∆Hfo) is in appendix of the text. The ∆Hfo values of most common compounds have been determined and tabulated. Standard Enthalpies of Formation CaCO3(s) has a ∆Hfo of -1207 kJ/mol. This is the enthalpy change for the reaction: Ca(s) + C(graphite) + 3/2 O2(g) CaCO3(s) Fractional coefficients are acceptable since all quantities are molar, and a formation reaction produces one mole of a compound. Standard Enthalpies of Formation ∆Hfo values can be used to calculate the standard enthalpy changes for many reactions. In an application of Hess’s Law, it is as if the reactants are decomposed into their elements, and then the elements are recombined into the desired products. Since enthalpies of reaction are independent of pathway, this provides an accurate way to calculate enthalpies of reactions. Standard Enthalpies of Formation CH4(g) + 2O2(g) CO2(g) + 2 H2O(l) Hess’s Law and ∆Hf o ∆Hrxno = ∑ ∆Hfo(products) - ∑ ∆Hfo(reactants) Problem: Use standard heats of formation to calculate ∆Horxn for the combustion of methane (CH4) : CH4(g) + 2 O2(g) 2 H2O(g) + CO2(g) Bond Dissociation Energies Bond dissociation energies can be used to estimate the enthalpy of a reaction. The enthalpy change will approximately equal the energy of bonds broken – energy of bonds formed. ΔHo ≈D(bonds broken) –D(bonds formed) Bond Energies Since bond energies are average values for a variety of molecules, they will only provide an estimate of the enthalpy change for a specific reaction. The following relationship can also be used to estimate enthalpies of reaction. ΔHo ≈D(reactant bonds) –D(product bonds) Bond Energies - Problem Use average bond enthalpies to estimate the enthalpy of reaction for: N2(g) + 3 H2(g) 2 NH3(g) Energetics of Ionic Bonds The lattice energy is a measure of the strength of ionic bonds within a specific crystal structure. It is usually defined as the energy change when a mole of a crystalline solid is formed from its gaseous ions. M+(g) + X-(g) MX(s) Lattice Energy Your text uses a different convention, defining the lattice energy as the energy change when a mole of a crystalline solid is converted to its gaseous ions. MX(s) M+(g) + X-(g) This approach results in positive values for lattice energy, and it requires energy to break apart and vaporize the ions in the solid. Lattice Energy Lattice energies cannot be measured directly, so they are obtained using Hess’ Law. They will vary greatly with ionic charge, and, to a lesser degree, with ionic size. Starting with the elements is their standard states, we know the enthalpies of sublimation, ionization, bond dissociation, electron affinity, and the enthalpy of formation for the ionic solid. 1/2 bond energy of Cl2 Electron Affinity of Cl Ionization energy of K ∆Hsub of K} ∆Hf of KCl –Lattice Energy of KCl Ionic charge has a huge effect on lattice energy. Ionic vs. Covalent Compounds Ionic Compounds Crystalline solids (made of ions) High melting and boiling points Conduct electricity when melted Many soluble in water but not in nonpolar liquid Covalent Compounds Gases, liquids, or solids (made of molecules) Low melting and boiling points Poor electrical conductors in all phases Many soluble in nonpolar liquids but not in water Properties of NaCl and CCl4