* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter Two Atoms & The Periodic Table
Heat exchanger wikipedia , lookup
Solar water heating wikipedia , lookup
Thermoregulation wikipedia , lookup
Intercooler wikipedia , lookup
R-value (insulation) wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Solar air conditioning wikipedia , lookup
Heat equation wikipedia , lookup
Cogeneration wikipedia , lookup
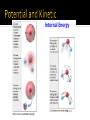
System: Part we care about Reactants & Products Surroundings: Everything else in the universe Aopen system (mass and heat pass through) Bclosed system (heat only pass through) Cisolated system (no heat or mass transfer) For chemical reactions to happen spontaneously, the final products must be more stable than the starting reactants Higher energetic substances are typically less stable and more reactive Lower energetic substances are typically more stable and less reactive Thermal energy flows from warmer to cooler H2O(s) H2O(l) 2H2(g) + O2(g) 2H2O(l) Study of heat and its transformations into other energies Thermochemistry is a part of this Thermodynamics studies changes in the state of a system State functions are properties that are determined by the state of the system, regardless of how it was achieved Only concerned with change, Δ ▪ Final – Initial Ex: ▪ Energy ▪ Pressure ▪ Volume ▪ Temperature Systems have a certain amount of internal energy Has 2 components: Kinetic energy: various types of molecular and electron motion Potential energy: attractive and repulsive interactions between atoms and molecules ΔU = U(products) – U(reactants) ΔU = q + w q = heat (absorbed or released by the system) w = work (done on or by the system) Calculate the overall change in internal energy (ΔU) for a system that absorbs 188 J of heat and does 141 J of work on its surroundings. Convert 723.01 J into calories SKETCH and LABEL what an exothermic and endothermic energy vs. time graph would look like. Calculate the overall change in internal energy for a system that releases 43 J in heat and has 37 J of work done on it by its surroundings Reactions can be carried out in two ways: In a closed container (constant volume): qv = ΔU (ΔU = q + w) In an open container (constant pressure): qp = ΔH (ΔH = ΔU + PΔV) work (w) heat (q) Enthalpy is the heat content absorbed or released in a system under constant pressure Combustion of propane gas: U ΔH = H(products) – H(reactants) “+” = endothermic “—” = exothermic H2O(s) H2O(l) ΔH = +6.01 kJ/mol CH4(g) + 2O2(g) CO2(g) + 2H2O(l) ΔH = -890.4 kJ/mol CH4(g) + 2O2(g) CO2(g) + 2H2O(l) ΔH = -890.4 kJ/mol How much energy is release from 18.40 g of methane being burned? If 924.3 kJ of energy was released, how many grams of water was produced? If you change the AMOUNTS in a balanced equation, you change the enthalpy the same way 1) Ex: if coefficients are doubled, so is the enthalpy ▪ ▪ 2H2(g) + O2(g) 2H2O(l) ΔH = -571.7 kJ 4H2(g) + 2O2(g) 4H2O(l) ΔH = -1143 kJ If you reverse the equation, you reverse the sign of the ΔH 2) Ex: 2H2(g) + O2(g) 2H2O(l) ΔH = -571.7 kJ 2H2O(l) 2H2(g) + O2(g) ΔH = +571.7 kJ How much energy is associated with burning 75.3 g of sulfur dioxide according to the following equation: What would be the energy associated with decomposing 1 mole of sulfur trioxide gas into sulfur dioxide gas and oxygen gas? (Hint: use properties of enthalpy!) Measurement or heat changes within a system Using a calorimeter vs. vs. Specific Heat (s): amount of heat required to raise the temperature of 1 g of a substance by 1°C (ex: liquid water is 4.184 J/(g·°C) q = (s)(m)(ΔT) q = heat (J); m=mass (g); ΔT=change in temp (oC) Heat Capacity (C): amount of heat required to raise the temperature of an object by 1°C (J/oC) q = (C)(ΔT) What is the amount of heat (in kJ) required to heat 255 g of water from 25.2 °C to 90.5 °C? 8540 J of energy is released when 927 g of granite is cooled. If the original temperature was 46.8 oC, what is the final temperature? Can calculate changes in heat using styrofoam cups Assuming constant pressure Therefore… qp = (m)(s)(ΔT) = ΔH A 30.4-g piece of unknown metal is heated up in a hot bath to a temperature of 92.4°C. The metal is then placed in a calorimeter containing 100. g of water at 25.0°C. After the calorimeter is capped, the temperature of the calorimeter raises to 27.2°C. What was the specific heat of the unknown metal? 125.0-g of a metal is heated to 100.0°C. It is then placed into a calorimeter containing 100.0 mL (100.0 g) of water at 25.0°C and capped. The energy is transferred and the max temperature of 34.1°C is reached. What is the specific heat of the metal? System: reactants and products (the reaction) Surroundings: water in calorimeter (resulting solution) For an exothermic reaction: The system loses heat The surroundings gain (absorb) heat Ex: 50.0 mL of 1.00 M HCl and 50.0 mL of 1.00 M NaOH are mixed in a calorimeter and capped at room temp (25.0°C). The reaction reaches a max of 31.7°C. What is the ΔH°rxn? CH4(g) + 2O2(g) CO2(g) + 2H2O(l) H2O (l) H2O(g) ∆H = -890.4 kJ/mol ∆H = +44.0 kJ/mol Given the following, determine the ΔH for 3H2(g) + O3(g) 3H2O(g) Standard Enthalpy of Formation (ΔH°f): heat change that results when 1 mole of a compound is formed from its constituent elements in their standard states “Standard State” means “stable form” at: 1 atm and 25°C Example: O(g) (249.4), O2(g) (0), O3(g) (142.2) ΔH°rxn: enthalpy of a reaction under standard conditions When we know reactions go to completion or can be done in one step, we can use a direct method Ex: Calculate ΔH°rxn for 2SO(g) + 2/3O3(g) 2SO2(g) From Appendix 2: SO(g): (5.01), O3(g): (142.2), SO2(g): (-296.4) Calculate the ΔHrxno for the following: CH4(g) + 2 O2(g) -> CO2(g) + 2 H2O(l) 2 H2S(g) + 3 O2(g) -> 2 H2O(l) + 2 SO2(g) When a reaction is too slow or side reactions occur, enthalpy of reaction can be calculated using Hess’s Law Recall: when bonds are made, energy is given off (exo); when bonds break, energy is needed (endo) Bond Enthalpy: the measure of stability of a molecule Enthalpy change associated with breaking a particular bond in 1 mole of gaseous molecules ▪ H2(g) H(g) + H(g) ΔH = 436.4 kJ/mol The higher the bond enthalpy, the stronger the bond The bonds in different compounds have different bond enthalpies Ex: O—H bond in water vs. O—H bond in methanol are different Therefore, we speak of AVERAGE bond enthalpy Recall: amount of energy required to convert 1 mole of ionic solid to its constituent ions in the gas phase Ex: NaCl(s) Na+(g) + Cl-(g)