* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Minerals
Survey
Document related concepts
Transcript
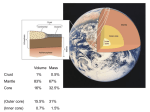
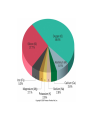
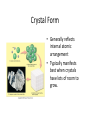
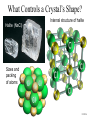
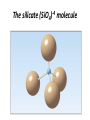
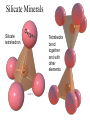
Atomic Structure and Minerals Basic Structure of Atoms Atoms • Smallest particles of matter • Have all the characteristics of an element Nucleus – central part of an atom that contains • _________ – positive electrical charges • _________ – neutral electrical charges Energy levels, or shells • Surround nucleus • Contain _________ – negative electrical charges Atomic Bonding and Isotopes Bonding of atoms • Forms a compound with two or more elements • Ions are atoms that gain or lose electrons Atomic number is the number of protons in an atom's nucleus _________ • Have varying number of _________ Isotopes • Have different mass numbers – the sum of the neutrons plus protons • Many isotopes are radioactive and emit energy and particles • C, O, U, Ar, K, and Pb are some of the most common isotopes used in geologic investigations. Periodic Table of the elements Average Abundances for the ____________ Hydrogen is most abundant Helium is second most abundant 04.10.b3 Average Abundances for the ____________ Oxygen second Magnesium is abundant metal 04.10.b2 abundant element Silicon third most abundant element Iron most (abundant) and nickel in core Sulfur abundant in core Average Abundances in _____________ Oxygen is most abundant element Some abundant metals Silicon is second most abundant; aluminum is third 04.10.b1 Iron is most abundant transition metal Elements in the Earth’s Crust • The eight elements that compose most rockforming minerals are oxygen (O), silicon (Si), aluminum (Al), iron (Fe), calcium (Ca), sodium (Na), potassium (K), and magnesium (Mg) • Most abundant atoms in Earth's crust are oxygen (46.6% by weight) and silicon (27.7% by weight) How Atoms Bond Together Sharing Loaning Free flow Metallic bond Covalent bond Stick together Intermolecular force Ionic bond 04.12.a How Are Atoms Arranged in a Mineral? 04.04.c1-3 Cubic Tetrahedron Octahedron Definition of a Mineral Definition of a mineral • _______ • _________ • _________ • Possess an orderly internal structure of atoms • Have a ________ chemical composition __________ - lacks an orderly internal structure Mineraloid Small openings Large openings Openings of different sizes allows the material to flow. Physical properties of minerals • • • • • • Crystal form Luster Color Streak Hardness Cleavage • Fracture • Specific gravity • Other properties • • • • Taste Smell Elasticity Malleability Crystal Form • Generally reflects internal atomic arrangement • Typically manifests best when crystals have lots of room to grow. What Controls a Crystal’s Shape? Internal structure of halite Halite (NaCl) Sizes and packing of atoms 04.04.a Crystal Lattice Orderly arrangement of atoms 04.04.b Repeating pattern Crystal Form Luster Metallic Non-metallic Color Streak Hardness Bonds with Same Strength Mineral can break along three sets of planes without passing through an atom 04.05.b Mineral breaks through the lattice in nearly any direction so it will fracture Cleavage Atomic Scale of Mineral Cleavage Brown atoms bonded with blue atoms into flat sheets (strong bonds) Sheets joined by long bonds between sheets (break along weakest bonds) Cleave into sheets 04.05.a F r a c t u r e Special Properties • Other properties • • • • Feel (Talc, Chlorite) Magnetism (Magnetite) Double Refraction (Calcite) Reaction to hydrochloric acid (Calcite) Building Rocks • A few dozen minerals are called the rockforming minerals • These minerals can be grouped according to their elemental makeup. Mineral Groups • _________ • Non-silicates – ___________ (Limestones) – Oxides – Sulfides – Native Metals– Elements – Sulfates Silicates • Composed of _________________ • Crystallize from molten material • Minerals are divided by how the silica tetrahedra are arranged. The silicate (SiO4)-4 molecule Silicate Minerals Silicate tetrahedron Tetrahedra bond together and with other elements 04.07.b Rock-forming silicates • Groups based upon tetrahedral arrangement • Olivine – independent tetrahedra • Pyroxene group – tetrahedra are arranged in chains • Amphibole group – tetrahedra are arranged in double chains Independent Tetrahedra 04.07.c Olivine Tetrahedra bond to other elements, not other tetrahedra Single Chains Tetrahedra bond together to form single chains Pyroxene 04.07.c Double Chains Tetrahedra bond to form double chains 04.07.c Amphibole Rock-forming silicates • Micas – tetrahedra are arranged in sheets • Two types of mica are biotite (dark) and muscovite (light) • Feldspars - Three-dimensional network of tetrahedra • Two types of feldspar are Orthoclase and Plagioclase • Feldspars are the most plentiful mineral group • Quartz – three-dimensional network of tetrahedra Sheet Silicates Mica 04.07.c Frameworks Tetrahedra bonded together and with other elements in 3D framework Quartz Feldspar 04.07.c










































![geol 1030 minerals [Compatibility Mode]](http://s1.studyres.com/store/data/003763777_1-ceb31933dd652b9e1f6873860c7f6491-150x150.png)