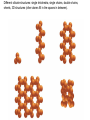* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download of minerals!
Survey
Document related concepts
Transcript
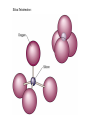
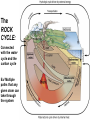
Volume Mass Crust 1% 0.5% Mantle 83% 67% Core 16% 32.5% (Outer core) 15.5% 31% (Inner core) 0.7% 1.5% Three Types of Rock: Igneous, Sedimentary, Metamorphic Rock: A solid, cohesive aggregate of grains of one or more MINERAL. Mineral: A naturally occurring, solid, inorganic element or compound, with a definite composition (or range of compositions), usually possessing a regular, internal crystalline structure. Three Types of Rock: Igneous, Sedimentary, Metamorphic Rock: A solid, cohesive aggregate of grains of one or more MINERAL. Mineral: A naturally occurring, solid, inorganic element or compound, with a definite composition (or range of compositions), usually possessing a regular, internal crystalline structure. Example: Rock = Granite Minerals = Quartz, Feldspars, Biotite, etc. Like states in a country, or countries in a continent. CRYSTAL - A mineral grain displaying the characteristics of its atomic structure. - almost 4000 different kinds of minerals - differences result from the different elements used and the ways they are bonded WHY DO MINERALS FORM? (Why not just separate elements?) Atoms are attracted - bond together to form large mega-molecules with same pattern crystal Atoms are constantly in motion, jumping from one structure to another Certain combinations of elements are stable If molecule is not stable, it breaks apart If molecule is stable, it continues to grow “Evolution” - survival of the fittest - of minerals! IONIC BOND Ex: Halite (salt) COVALENT BOND Ex: Diamond METALLIC BOND: Electrons are free to move about between atoms For a Mineral to be Stable: 1. Ionic charges sum to ZERO 2. Ion sizes must be compatible (sizes determined by electron cloud) Imagine what happens in a cooling magma chamber: Like a game of musical chairs with the atoms “sitting down” into growing crystals Early Earth Most rocks are variations on silicon and oxygen: silicates BULK COMPOSITION OF THE EARTH Element Iron (Fe) Oxygen (O) Silicon (Si) Magnesium (Mg) Nickel (Ni) Sulfur (S) Calcium (Ca) Aluminum (Al) % Weight 35 30 15 13 2.4 1.9 1.1 1.1 COMPOSITION OF THE EARTH'S CRUST Element Oxygen (O) Silicon (Si) Aluminum (Al) Iron (Fe) Sodium (Na) Calcium (Ca) Magnesium (Mg) Potassium (K) % Atoms 62.6 21.2 6.5 1.9 2.6 1.9 1.8 1.4 % Weight 46.6 27.7 8.1 5.0 2.8 3.6 2.1 2.6 % Volume 91.7 0.2 0.5 0.5 2.2 1.5 0.4 3.1 Silica Tetrahedron: Different silicate structures: single tetrahedra, single chains, double chains, sheets, 3D structures (other atoms fill in the spaces in between). Slightly changing the different elements that combine with silica greatly changes the mineral that results, or the characteristics of the mineral. Ex/ Different forms of quartz The ROCK CYCLE: Connected with the water cycle and the carbon cycle Ex/ Multiple paths that any given atom can take through the system