* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download carbapenam and monobactam - Home
Discovery and development of proton pump inhibitors wikipedia , lookup
Development of analogs of thalidomide wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Pharmacognosy wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Drug discovery wikipedia , lookup
Discovery and development of tubulin inhibitors wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Environmental persistent pharmaceutical pollutant wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Antibiotics wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
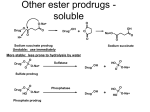
Carbapenems Carbapenems are: - β-lactams that contain a fused β-lactam ring and a 5-membered ring system that differs from the penicillins in being unsaturated (double bond between C-2 and C-3) and containing a carbon atom instead of the sulfar atom. OH H H H S HC H3C R N N COOH Carbapenem H S R O H N O CH3 CH3 COOH Penicillin Thienamycin Thienamycin isolated from fermentation of cultures of Streptomyces cattleya. OH H H S H3C NH3+ O N Thienamycin O- O An unfortunate property of thienamycin is its chemical instability (unstable) in solution. Another shortcoming is its susceptibility to hydrolytic inactivation by renal dehydropeptidase-I (DHP-I). Imipenem Imipenem is N-formimidoylthienamycin, the most stable derivatives of thienamycin. HN OH H H NH S H3C N O O Imipenem OH Mechanism of action Imipenem like other β-lactam antibiotics binds to penicillinbinding proteins, disrupts bacterial cell wall synthesis and cause death of susceptible micro-organisms. Structure activity relationship OH H H 4 H3C 1 O S 3 NH3+ 2 N Thienamycin O- O HN OH H3C H H 4 1 N O 3 NH S 2 O Imipenem OH Uses: - Aerobic Gm +ve organisms such as S. aureus, Enterococci and Streptococci - Aerobic Gm –ve bacteria such as E. coli, Klebsiella and Enterobacter spp. and P. aeruginosa. - Anaerobes such as B. fragilis and Clostridium. Poor distribution in CSF (not used in meningitis) Pharmacokinetics Adverse reactions Investigational carbapenems The extended spectrum of antibacterial activity associated with the carbapenems together with their resistance to inactivation by most β-lactamase make this class of β-lactamas an attractive target for drug development. In the design new carbapenems, structural variations are being investigated with the objective of developing analogues with advantages over imipenem. Improvements that are particularly desired include: - Stability to hydrolysis catalysed by DHP-I. - Increased potency against Ps. aeruginosa. - Enhanced pharmacokinetic properties, such as oral bioavailability and a longer duration of action. Modification by: - Incorporation of a β-methyl group at the 4-position confers stability of the carbapenems to hydrolysis by renal DHP-I. - Substituents at the 3-position appear to affect primarily the spectrum of antibacterial activity of the carbapenems by influencing their penetration into bacteria. Meropenem OH H H 4 H3C O CH3 3 S O 2 N1 HO Meropenem O N H N CH3 H3C Meropenem is a second generation carbapenem. Meropenem is not hydrolyzed by DHP-I and is resistant to most β-lactamases, including a few carbapenemases that hydrolyze carbapenem. The lower incidence of nephrotoxicity of meropenem (compared with imipenem) has been correlated with its greater stability to DHP-I. Biapenem OH H H CH3 + N S H3C N N N O -O O Biapenem Biapenem is a second generation carbapenem with chemical and microbiological properties similar to those of meropenem. Biapenem is stable to DHP-I and is resistant to most β-lactamases. Monobactam Monobactams have a monocyclic β-lactam ring and are resistant to β-lactamases. R O H C N H H CH3 N O Monobactam SO3H Aztreonam S H2N N C O H C N N N Aztreonam CH3 CH3 O O C COOH SO3H CH3 Aztreonam was isolated from Chromobacterium violaceum . Aztreonam is the first clinically useful monobactam. The antimicrobial activity of Aztreonam differs from those of other β-lactam antibiotics and more closely resembles that of an aminoglycosides in activity without the nephrotoxicity of aminoglycosides. Mechanism of action Uses: Aztreonam is unique among the β-lactam antibiotics b/c it is active only against Gm–ve aerobes and inactive against Gm +ve and anaerobes. The combination of Aztreonam and piperacillin is synergistic against some strains of P. aeruginosa and Enterobacter spp. Pharmacokinetics: 1- Absorption 2- Excretion 3- Penetration/distribution Toxicity It is a safe agent with side effect similar to those of other β-lactams. Adverse effects Tigemonam S H2N O N C C H N Aztreonam CH3 H2N N N O C O COOH S CH3 N SO3H C O H C N CH3 CH3 N N Tigemonam CH3 O O SO3H CH2COOH It is an investigational monobactam that is orally active. It is highly resistant to β-lactamases. The antibacterial spectrum of activity of tigemonam resembles that of aztreonam. It is very active against the Enterobacteriaceae, including: E. coli, Klebsiella, Proteus, Enterobacter species.