* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Alpha – 1 Antitrypsin (AAT) Deficiency
Survey
Document related concepts
Transcript
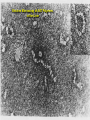
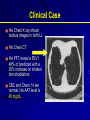
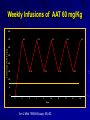
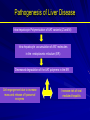
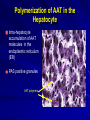
Alpha – 1 Antitrypsin Deficiency Jorge Mera, MD Presbyterian Hospital of Dallas Alpha – 1 – Antitrypsin Deficiency Mechanism of Alpha-1- Antitrypsin Deficiency (AATD) Clinical Case (Presentation) Lung Disease – Pathogenesis – Clinical Presentation – Treatment Extra-Pulmonary Disease – Hepatic Disease Pathogenesis Clinical Presentation – Other Clinical Case (Resolution) Serpin These are inhibitors of proteolytic enzymes with a serine residue at the active site – AAT, Antithrombin, C1-inhibitor and alpha 1 antichymotrypsin When they bind to its target proteinase it undergoes a conformational change The advantage – Is that the conformational change stabilizes the complex – It allows the modulation of inhibitory activity The disadvantage of conformational mobility is their vulnerability to mutations which can : – Decrease its activity – Allow inappropriate changes that lead to polymerizations Protein Folding and Function Elastase AAT AAT AATD is a Protein Folding Disease Protein folding is the process by which an unfolded polypeptide chain folds in to a specific native and functional structure Defective protein folding is an important mechanism underlying the pathogenesis of many diseases Protein Folding and Disease Disease Protein Affected Molecular Defect Cystic fibrosis transmembrane regulator (CFFTR) Misfolding and retention in the ER, leading to degradation Marfan Syndrome Fibrillin Misfolding Nephrogenic Diabetes Insipidus Vasopressin receptor or aquaporin water channel Misfolding and retention in the ER Alfa -1- Antitrypsin Deficiency Alfa -1- Antitrypsin Misfolding and retention in the ER leading to aggregation in cells of synthesis Creutzfeldt-Jakob Disease Prion protein Aggregation in brain (after protein release) Alzheimer’s Disease Beta-amyloid Aggregation in brain (after protein release) Cystic Fibrosis ER: Endoplasmic Reticulum Abnormal Folding and Polymerization of AAT The most common and severe form of AAT deficiency is caused by e Z mutation, a single base substitution (Glu342-lys) in the AAT gene. This slows the rate of protein folding in the cell Allowing the accumulation of an intermediate which polymerizes Impeeding its release Leading to plasma deficiency AAT Polymer Electron Microscopy of AAT Polymers in the Liver Clinical Case CC: 45 yowm comes to your office with a CC of Dyspnea on mild exercise. PMH: Is unremarkable, and he never smoked Family Hx: His Father was a smoker and died of Emphysema at 43 years of age and his mother is 73 yo and in good health. He has 2 sons 19 and 21 years old, his older son has a 3 pack/year smoking Hx and the 19 yo has IgA deficiency PE: Vital signs reveal BP 120/74 HR: 88 RR: 20/min. The only positive findings are diminished bilateral breath sounds and an emphysematous type Chest wall. Clinical Case His Chest X ray shows bullous images in both LL His Chest CT His PFT reveal a FEV1 48% of predicted with a 35% increase on inhaled bronchodilators. CBC and Chem 14 are normal. His AAT level is 45 mg/dL. Clinical Case Does he have a AAT deficiency ? What other tests should you order? What is his prognosis? What information regarding treatment should you give him? Is he a candidate for AAT augmentation therapy? If so, what precautions should you take before starting treatment? Should his siblings be tested for AATD and Phenotype? What will you do with the results if they are abnormal? AATD Described in 1963 by Laurell and Erikson1 Underrecognized Disorder that may affect – Lungs – Liver – Skin (rarely) AAT – Inhibitor of proteolytic enzyme elastase – Member of the Serpins Family (Serine Protease Inhibitors) – 90 Alleles Identified 1. Laurell, C-B,Eriksson, A. Scand J Clin Lab Invest 1963: 15:32 AATD Lung Disease AAT Phenotypes Normal Deficient Null Dysfunctional Phenotype AAT Levels AAT Function MM Normal Normal. ZZ (most common) Under 35 % of normal level Normal Null Null 0% NA varies Normal Abnormal What is the minimum Level of AAT necessary for lung protection? 11 umol/L or 80 mg/dL (NV: 20-53 umol/L or 150 300 mg/dL) Based on population studies Pathogenesis of Lung Damage in AATD AAT Clinical Case Epidemiology USA: Worldwide 80,000 – 100,000 3,000,000 Worldwide racial and ethnic distribution of alpha(1)-antitrypsin deficiency. Chest 2002;122:1818 Prevalence Based on a US population of 250 million – COPD screening1: 2 - 3 % of 965 COPD patients screened1 If in the USA there are 2.1 million patients with Emphysema, 40,000-60,000 would be expected to be AAT Deficient – Direct population screening studies2 1:1575 – 1:5097 are positive 80,000 - 100,000 would be expected to be AAT Deficient 1. Chest 1986;89:370 2. N Eng J Med 1976;294:1316 Why is AAT Deficiency Underdetected ? Many patients are asymptomatic despite severe deficiency Lack of recognition of symptomatic patients by physicians – In a cohort of 304 AAT deficient patients Mean time to diagnosis was 7.2 years Number of physicians seen before diagnosis was made – 3 (43% of the patients) – 6 – 10 (12 % of the patients) Cleve Clin J Med 1994;61:461 Why is it important to detect AATD Treatment is available Counseling – Of the patient to avoid other risk factors – Of the siblings for screening and Clinical Presentation Emphysema – Pathogenesis: Imbalance between neutrophil elastase in the lung which destroys elaste and elastase inhibitor AAT which protects against proteolytic degradation of elastin – Risk factors: Phenotypes associated with a AAT levels below the “Protective threshold” of 11umol/L Smoking Parental Hx of o COPD Bronchiectasis ? Asthma ? Lung Related Clinical Manifestations Emphysema – Presenting Symptoms: Dyspnea (most common symptom) Cough, phlegm production and wheezing Bronchodilator responsiveness – Differences with patients w usual COPD Earlier Age Bullous changes prominent in lung bases – > 90 % of ZZ phenotype have lung bases involved – Limited to lung bases in 24 %Found exclusively in Asthma and Bronchiectasis: – Relationship not proven Diagnosis Measure AAT level Phenotype by isoelectric focusing Genotype AAT Phenotypes Phenotype Risk for Emphysema True Plasma level (umol/L) Commercial Standard (mg/dL) MM No increase 20-53 150-350 MZ Possible mild increase 12-35 90-210 SS No increase 15-30 100-140 SZ Mild Increase (20 -50%) 8-19 75-120 ZZ High Risk (80 – 100%) 2.5-7 20-45 Null High Risk (100% by age 30) 0 0 Am Rev Respir Dis 1989;140:1494 Risk for developing lung disease Smoking: – Age of onset of Dyspnea in AAT (ZZ) Deficient Non-smokers Vs Smoker1: 32 - 40 vs 48 – 54 – In heterozygous SZ phenotype, COPD rarely occurs unless smoking is present2 Family History: – MZ phenotypes have increased risk of COPD only when they have a symptomatic first degree relative3 Airway irritants: – Risk of other irritants in disease progression is controversial 1. Lancet 1985;1:152 2. Am J Respir Crit Care Med 1996;154:1718 3. Am J Respir Crit Care Med 2000;161:81 Survival in AAT according to FEV1 0.6 0.5 0.4 Mortality rate 0.3 0.2 0.1 0 15 20 25 30 35 % of predicted FEV 1 2 year Mortality Seersholm N et al. Eur Respir J 1994;7:1985 60 Treatment Augmentation Therapy – Intravenous (only one FDA approved) – Aerosolized Enhancement of endogenous AAT Gene Therapy IV Augmentation Therapy FDA approved IV AAT based on clinical studies that proved that the infusion: – Increase plasma and ELF levels of AAT – Increase Levels anti-neutrophil elastase activity in ELF recovered by BAL – Is Safe and well tolerated There are no randomized clinical trials that prove clinical efficacy in change in natural history of emphysema Indication of IV AAT is based on observational studies IV Augmentation Therapy: Concerns The true protective threshold value (AAT level) – Is not available – It is estimated from values that separate affected from unaffected individuals Some severely deficient patients have normal lung function – Plasma levels alone do not predict disease they only assign risk The proportion of individuals with ZZ phenotype that do not develop clinically significant emphysema is not known Observational Studies National Registry of Patients with Severe AATD conducted a prospective cohort study1 – Survival was enhanced in recipients of augmentation therapy – The subset with FEV1 35 % – 49 % of predicted had a slower decline of FEV1 over time Study comparing Ex- German Smokers (198) with treatment (3.2 years) with Ex Danish smokers (98) without treatment2 – Lower FEV1 decline in treatment group (53ml vs 75ml per year, P= 02) Study evaluating 96 patients with severe AAT before and after treatment3 – Showed a lower FEV1 only in those with mild airflow obstruction 1. Am J Respir Crit Care Med 1998;158:49. 2. Eur Respir J 1997;10:2260 3. Chest 2001;119:737 Selection Criteria for Treatment High – risk phenotype (ZZ or Null) Plasma AAT level below 11 umol/L Airflow obstruction by Spirometry – American Thoracic Society: < 80 % of predicted – Canadian Thoracic Society: 35% - 50 % of predicted Patient compliance to treatment Age equal to or greater than 18 Nonsmoker or ex-smoker Selection Criteria for Treatment Not recommended for – Heterozygous Phenotypes – AAT > 11umol/L Unknown – Fixed severe obstruction – Normal airflow but radiographic evidence of Emphysema Goals of IV Infusion Maintain a through level above the protective threshold Diffusion of AAT in lung tissue (ELF) In vivo anti neutrophil elastase activity after infusion AAT Infusion: Side Effects Low grade self limited fever Anaphylaxis with IgE antibody formation to AAT (rare) Syndrome of – Transient fever – Chest and low back pain Biological hazard Anaphylaxis in IgA deficient patients Weekly Infusions of AAT 60 mg/Kg 400 350 350 350 350 350 350 Concentration of AAT in mg/dL 300 250 200 150 150 150 150 150 100 50 0 0 -7 0 2 7 9 14 Days Am J Med 1988;84(supp; 6A):52. 16 21 23 28 Monthly Infusion of 250mg/Kg of AAT ELF Antitrypsin Activity 3.5 ELF AAT Level, umol 3 2.5 Patient 1 Patient 2 Patient 3 2 1.5 1 0.5 0 0 1 2 3 AAT protective level 4 5 6 7 8 9 10 Months JAMA 1988;260:1259 ELF antielastase capacity, mmol Efficacy of aerosolized AAT 6 5 4 3 2 Normal range 1 0 1 2 3 4 5 6 7 Days Line 1 Hubbard, RC et al. Ann Intern Med 1989;111:206 8 Management of Candidates for Augmentation Therapy Pre- Treatment Testing – Respiratory Function: Spirometry DLCO Supportive Therapy – Cessation Smoking Respiratory irritants – Non-Specific Treatments – Laboratory: Hepatitis profile LFT’s HIV Titer – Immunization Hepatitis B vaccine IVIG immunoglobulin Bronchodilators Pulmonary Rehab Oxygen Therapy Early treatment of respiratory infections – Vaccines: Pneumococcal Influenza Extra-pulmonary AAT Deficiency Hepatic Disease (most frequent) Skin Disease – Panniculitis (1:1000 of AATD) More inflammatory More “oily discharge” More acute inflammation in histology Vascular disease – Aneurysms – Fibromuscular displasia – AAT Pittsburg mimics effects of antithrombin III Glomerulonephritis – Prolipherative GN – IgA GN Inflammatory Bowel disease – AATD patients have more severe Colitis Hepatic Disease Liver Diseases associated with AAT phenotypes Neonatal hepatitis Elevated transaminases in young adults Cirrhosis in children and adults Hepatocellular carcinoma “Null” Phenotype has no risk of hepatic disease and a High risk of Emphysema Pathogenesis of Liver Disease Intra-hepatocyte Polymerization of AAT variants (Z and M) Intra-hepatocyte accumulation of AAT molecules in the endoplasmic reticulum (ER) Decreased degradation of the AAT polymers in the ER Cell engorgement due to increase mass and release of lysosomal enzymes Increase risk of viral mediated hepatitis Polymerization of AAT in the Hepatocyte Intra-hepatocyte accumulation of AAT molecules in the endoplasmic reticulum (ER) PAS positive granules AAT polymers Natural History of Hepatic Disease of ZZ Phenotype 0% 20% 40% Neonatal Disease 60% 80% 100% Adulthood Disease Free of Disease Natural History of Hepatic Disease 15 % neonatal hepatitis – 5% Cirrhosis in the 1st year of life – 10 % 25% Resolution of hepatitis by ages 3 to 10 25% Cirrhosis between age 6 mo and 17 years 25% Histological evidence of cirrhosis with survival through the first decade 25% Elevated LFT’s without Cirrhosis 85% Asymptomatic at childhood – Cirrhosis in 11.8 % – Hepatocellular carcinoma in 3.3 % – 85% No Disease Clinical Case Resolution Does he have a AAT deficiency ? – YES What other tests should you order? – PHENOTYPE ZZ What is his prognosis? – According to FEV1 15 % in 2 years What information regarding treatment should you give him? – That he is a candidate for Augmentation therapy but that there are no clinical trials to assure him improvement Is he a candidate for AAT augmentation therapy? – Yes, his age, FEV1, AAT level and phenotype and non-smoker status making him a good candidate Clinical Case Resolution If so, what precautions should you take before starting treatment? – Hep B vaccination, HIV testing, Influenza and Pneumococcal vaccines Should his siblings be tested for AATD and Phenotype? – Yes What will you do with the results if they are abnormal? – His 21 year old son is ZZ phenotype, FEV1 is normal Stop smoking and control of FEV1 – His 19 year old son is ZM phenotype (probably like his mother) and FEV1 is also normal Avoid smoking No treatment warranted since AAT infusion can cause anaphylaxis in IgA deficiency Situations to Suspect Severe Deficiency of AAT Emphysema in a young individual (less than 45 years old) Emphysema in a non smoker Emphysema characterized by predominant basilar changes on the chest x-ray Family History of Emphysema and/or liver disease (unexplained cirrhosis or hepatoma) Clinical findings or history of panniculitis Clinical findings or history of unexplained chronic liver disease THANK YOU