* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 04. Physical-chemical essence of surface phenomenon
Survey
Document related concepts
State of matter wikipedia , lookup
Flux (metallurgy) wikipedia , lookup
Energy applications of nanotechnology wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Tunable metamaterial wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
Nanochemistry wikipedia , lookup
Centrifugal micro-fluidic biochip wikipedia , lookup
Self-assembled monolayer wikipedia , lookup
Ultrahydrophobicity wikipedia , lookup
Transcript
LECTURE 4:
Physical-chemical essence of
surface phenomenon.
ass. prof. Yevgeniya B. Dmukhalska
• Surface tension is the force or tension required
•
•
to break film and is defined as the force in dynes
acting upon а line one cm long on the surface of
the liquid.
Surface tension is typically measured in
dynes/cm, the force in dynes required to break a
film of length 1 cm. Equivalently, it can be stated
as surface energy in ergs per square centimeter.
Water at 20°C has a surface tension of 72.8
dynes/cm compared to 22.3 for ethyl alcohol
and 465 for mercury.
Drop method
When а liquid is allowed to flow very slowly through а capillary
tube, а drop will form which will increase to а certain size and
then fall down. For finding out the surface tension of а liquid
relative to that of water, the number of drops produced by а
given volume of the two liquids is found out. The apparatus
used in these determinations consists of а bulb fused with а
capillary tube and is called а drop pipet or stalagmometer
liquid = number of drops of water x water /number of drops of liquid
• Stalagmometer
Using torsion balance or
tensiometer (Du Nouy’ s).
In this method а light metal ring is set on
the surface of the liquid. When the ring is
raised, а film of the liquid clings to it. The
amount of force required to pull the ring
and break the film is measured and gives
the surface tension.
Surfactants
• Class of molecules that contain hydrophobic
•
•
•
(non-polar) hydrocarbon "tails" and a hydrophilic
(polar) "head" group are called surfactants.
А surfactant accumulates at the interface, and
modifies its surface tension.
Is а surface which separates а liquid from air or
other gases or which separates one liquid from
another.
Soaps, detergents, bile salts and proteins
If the material is hydrophilic ("water loving") it has a
surface to which water is attracted.
If the solid object is hydrophobic ("water fearing"),the
unfavorable interactions between the water surface and
the object make it difficult to wet the surface.
• The surfactant molecules thereby organize
themselves into 3-dimensional spheres called
micelles which have a hydrocarbon core and
polar groups around the outer surface.
• Some surfactants can coat the surface of the
water to form a layer one molecule thick, a
molecular monolayer.
• The phenomenon of attracting and retaining the
molecules of а substance on the surface of а liquid
or а solid resulting into a higher concentration of the
molecules on the surface is called adsorption.
• The substance thus adsorbed on the surface is
called the adsorbate and the substance on which it
is adsorbed is called adsorbent. The reverse process
removal of the adsorbed substance from the surface
is called desorption.
• The adsorption of gases on the surface of metals is
called occlusion.
• The process of adsorption involves separation of a
substance from one phase accompanied by its
accumulation or concentration at the surface of
another.
Adsorption:
• It is а surface phenomenon i.е. it occurs only at
the surface of the adsorbent.
• In this phenomenon, the concentration on the
surface of adsorbent is different from that in the
bulk.
• Its rate is high in the beginning and then
decreases till equilibrium is attained.
Absorption:
• It is а bulk phenomenon i.e. occurs throughout
the body of the material.
• In this phenomenon, the concentration is same
throughout the material.
• Its rate remains same throughout the process.
• When the concentration of the adsorbate
is more on the surface of the adsorbent
than in the bulk. it is called positive
adsorption.
• If the concentration of the adsorbate is
less relative to its concentration in the
bulk, it is called negative adsorption.
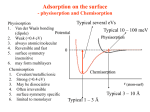
Physical adsorption:
1. The forces operating in these cases are weak van-derWaal’s forces.
2. The heats of adsorption are low viz. about 20 – 40
kJ/mol
3. No compound formation takes place in these cases.
4. The process is reversible i.е. desorption of the gas
occurs by increasing the temperature or decreasing the
pressure.
5. It does not require any а activation energy.
б. This type of adsorption decreases with increase of
temperature.
7. It is not specific in nature i.е. all gases are adsorbed on
all solids to some extent.
8. The amount of the gas adsorbed is related to the ease of
liquefaction of the gas.
9. It forms multimolecular layer.
Chemisorption:
1. The forces operating in these cases are similar
to those of а chemical bond.
2. The heats of adsorption are high viz. about
400-400 kJ/mol
3. Surface compounds are formed.
van der Waals Bonding
• dipole moment
• dipole electric field
• Acidic or cation exchange resins – These contain
•
•
acid groups, е.g. sulfonic acid (SO3H), carboxyl group
(СООН) or phenol group (ОН). One example is Dowex50. Their acid groups dissociate as do other acid groups.
For example Resin.СООН which may also be written as
Resin-Н+ will dissociate as Resin- Н+ Resin-+ Н+.
These resins may occur as free acids or as one of their
salts, е.g. Resins-.Na+. An example of the use of а salt of
а cation exchange resin is given below:
2(Resin- .Na+.) + Ca2+ (Resin-)2Са2+ + 2Na+
• Anion exchange resins - These contain а
•
weakly basic group like – NH2 or а strongly basic
quaternary ammonium group - (NR3)+. One
е,сатр1е is Dowex-1. These resins can bind
negatively charged groups like hydroxyl, halide,
citrate, su1fate, etc. An example of the use of
anion exchange resin in medicine is its
administration by mouth to bind gastric HCl in
the treatment of peptic ulcer.
Resin+ ОН- + НС1 Resin+Cl- + Н2O
Uses of ion exchange resins
• 1. Removal of excess of Na+ and К+ from body
•
•
•
•
fluids in congestive heart failure and renal failure
respectivly; а cation exchange resin is given by
mouth or by enema.
2.Production of low sodium milk for special
dietary needs.
3. Removal of radioactive Sr90 from milk of cows
feeding on pastures containing Sr90
4. In the separation and purification of amino
acids, vitamins and. hormones.
5. А very important technique based on the
selective adsorption of chemical compounds by
various ion exchange resins at specific pH values
is called column chromatography.