* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1. Introduction to Natural Products Chemistry
Metalloprotein wikipedia , lookup
Point mutation wikipedia , lookup
Peptide synthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Genetic code wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Biochemistry wikipedia , lookup
Amino acid synthesis wikipedia , lookup
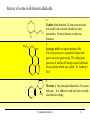
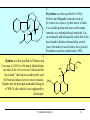
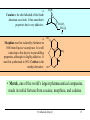
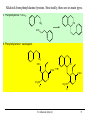
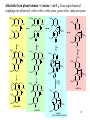
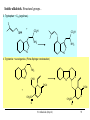
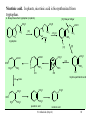
9.0 Alkaloids RA Macahig FM Dayrit H HO N HO O H H H NCH3 CH3O Quinine HO Morphine N Introduction • Among all the groups of natural products, alkaloids have the most colorful history, having achieved the most fame and notoriety as drugs. Where alkaloids occur, they tend to dominate the biological activity. Despite their relatively limited distribution, the alkaloids probably have the most significant impact in human history particularly in medicine, social issues, economics and politics. • In 1819, Carl Friedrich Meissner, a pioneering German pharmacist, coined the term “alkaloid” which referred to any natural product with the characteristic presence of the basic nitrogen atom, excluding peptides. (Amides, however, are generally included.) 9.0 Alkaloids (Dayrit) 2 Introduction • Because many alkaloids can be purified from crude extracts by acid-base extraction and recrystallization, these were the first natural products to be purified, characterized and commercialized. • The powerful and immediate effects of alkaloids are thought to be due to the presence of the cationizable N-atom which gives it lipid- and water-soluble characteristics, and enables it to cross membrane barriers more readily. 9.0 Alkaloids (Dayrit) 3 Introduction • Many of the earliest pure compounds to be used as drugs developed were alkaloids: • Cocaine: anaesthetic, from South American Erythroxylum coca • Quinine: antimalarial, from the bark of the Cinchona tree • Morphine: anaesthetic, from opium (Papaver somniferum) • Emetine: for amoebiasis, from ipecac, the powdered roots (Cephaelis species) • Strychnine: poison, from the seeds of Strychnos nux-vomica • The alkaloids have a relatively limited distribution in nature compared with the other natural product groups. Alkaloids were originally thought to be uniquely plant products until the 1950s when several alkaloids were isolated from bacteria, fungi and algae, insects, and amphibians. • A number of fungi produce toxic alkaloids, notably Claviceps purpurea. 9.0 Alkaloids (Dayrit) 4 Introduction • In the plant kingdom, the angiosperms produce alkaloids: Apocynaceae, Papaveraceae, Rubiaceae, Ranunculaceae, Solanaceae, and Berberidaceae. Among the monocots, only the Amaryllidaeae and Liliaceae produce alkaloids. (cms.herbalgram.org) • The discovery of many alkaloids are associated with anthropological explorations. Here, a Kamsá youth from a Brazilian tribe holds a blossom of Culebra borrachera which is a toxic psychoactive plant. It contains tropane alkaloids that can induce a frightening state of psychotic delirium, and ultimately stupor and death. 9.0 Alkaloids (Dayrit) 5 Introduction • Some animals, notably some soft corals and frogs produce highly bioactive alkaloids. In some cases, however, the alkaloids were found to have been ingested in the diet by the organism and then modified for use. Well-known examples are the pyrrolizidine alkaloids in caterpillars and moths. Alkaloids are much less common in mammals. Intermedine (I) and lycopsamine (II) ingested from the leaves of Mikania scandens 9.0 Alkaloids (Dayrit) monocrotaline (III) 6 Introduction • There are four major groups of nitrogen-containing organic compounds in biological systems: the amino acids (peptides and proteins), the nucleoside bases (DNA and RNA), the porphyrins; and the alkaloids. The first 3 groups are primary metabolites; the alkaloids are secondary metabolites. • Alkaloids are defined simply as nitrogen-containing natural products. In terms of chemical structure, alkaloids can be classified into the following: 2, 3, and 4 alkyl amines; and heterocyclic amines (e.g., pyrrolidine, pyridine, indole, quinoline, and isoquinoline). N pyrrolidine N pyridine N N indole N quinoline 9.0 Alkaloids (Dayrit) isoquinoline 7 • Ajmaline, antiarrythmic that functions by inhibition of glucose uptake by heart tissue mitochondria • Atropine (hyoscyamine), anticholinergic, antidote to nerve gas poisoning • Caffeine, widely used central nervous system stimulant • Camptothecin, potent anticancer agent • Cocaine, topical anesthetic, potent central nervous system stimulant, and adrenergic blocking agent, drug of abuse (Kutchan, The Plant Cell, 7, 1059-1070, July 1995) • Codeine, relatively nonaddictive analgesic and antitussive; Coniine, first alkaloid to be synthesized, extremely toxic, causes paralysis of motor nerve endings, used in homeopathy • Emetine, orally active emetic, amoebicide; morphine, powerful narcotic analgesic, addictive drug of abuse; Nicotine, highly toxic, causes respiratory paralysis, horticultural insecticide • Pilocarpine, peripheral stimulant of the parasympathetic system, used to treat glaucoma • Quinine, traditional antimalarial, important in treating Plasmodium falcipafum strains that are resistant 9 to other antimalarials • Sanguinarine, antibacterial showing antiplaque activity, used in toothpastes and oral rinses • Scopolamine, powerful narcotic, used as a sedative for motion sickness • Strychnine, violent tetanic poison, rat poison, used in homeopathy; Taxol, antitumor agent • (+)-Tubocurarine, nondepolarizing muscle relaxant producing paralysis, adjuvant to anesthesia • Vinblastine, antineoplastic that is used to treat Hodgkin’s disease and other lymphomas. 10 Survey of some well-known alkaloids. Coniine from hemlock (Conium maculatum) was used by the ancient Greeks for state executions. Its most famous victim was Socrates. Lysergic acid is an opiate produced by Claviceps purpurea, a parasitic fungus that grows on some grain crops. The unhygienic practices of medieval Europe caused outbreaks of convulsion which was called “St. Anthony’s Fire”. N H HO2C NCH3 N N CH3 N Nicotine is the principal alkaloid in Nicotiana tabacum. It is addictive and has been recently classified as a drug. 9.0 Alkaloids (Dayrit) 11 N H N O H H O Quinine was first purified by Pelletier and Caventou in 1820. It is the major alkaloid from the bark of the Cinchona tree. Christened the “Jesuit bark,” the bark was traditionally used by Peruvian indians for fever due to malaria. Quinine was the principal antimalarial drug up to WW II, after which it was supplanted by chloroquin. Strychnine was first purified in 1818 by Pelletier and Magendie from the seeds of Strychnos nux-vomica, a plant native to India. It is a deadly poison and was used for many centuries as a rodenticide and vermicide. It is so chemically and biologically stable that it has been found in bodies exhumed after several years (this makes it a bad choice for a poison!) Strychnine was first synthesized in 1954. N HO H H CH3O 9.0 Alkaloids (Dayrit) N 12 Cocaine is the chief alkaloid of the South American coca bush. It has anaesthetic properties but is very addictive. CH3N CO2CH3 OCC6H5 O Morphine was first isolated by Sertürner in 1806 from Papaver somniferum. It is still valued up to this day for its pain-killing properties, although it is highly addictive. It was first synthesized in 1952. Codeine is the methyl derivative. HO NCH3 O H HO • Merck, one of the world’s largest pharmaceutical companies, made its initial fortune from cocaine, morphine, and codeine. 9.0 Alkaloids (Dayrit) 13 Mescaline is a well-known hallucinogenic compound extracted from the Indian peyote cactus, Laphophora williamsii. CH3O NH2 CH3O CH3O O H3C O CH3 N N N N CH3 Caffeine is perhaps one of the most widely consumed alkaloids in the world. It is a member of a group of xanthine alkaloids which is present in coffee. The other famous xanthines, theobromine and theophylline, are found in tea and cocoa, respectively. Vinblastine is the anticancer drug from Catharanthus roseus (locally known as chichirica). Because it occurs in very minute amounts in the plant, this plant has been the subject of intense biotechnology research to try to produce it in vitro using cell culture techniques. N N CH3OC O CH3O HO N N OCCH3 CH3OC 9.0 Alkaloids (Dayrit) O OH O 14 Introduction • Many alkaloids are toxic in the cytoplasm, even in the plants that produce them. Their accumulation, therefore, requires a well-regulated and compartmentalized system. The most common storage organ is the vacuole. • In some plants, alkaloids are synthesized in one part of the plant and then are transported to another part for storage. In tobacco, for example, alkaloids (e.g., nicotine) are synthesized in the roots and are then transported via the xylem to the leaves where they are accumulated. 9.0 Alkaloids (Dayrit) 15 Introduction Among the natural products groups, the biochemistry of alkaloids is the most complex. • Many alkaloids are mixed metabolites. • Enzymes involved in alkaloid biosynthesis are associated with diverse subcellular compartments including the cytosol, vacuole, tonoplast membrane, endoplasmic reticulum, chloroplast stroma, thylakoid membranes, and perhaps unique biosynthetic or transport vesicles. • Localization studies have shown that sequential alkaloid biosynthetic enzymes can also occur in distinct cell types, suggesting the intercellular transport of pathway intermediates. (PJ Facchini, Ann. Rev. of Plant Physiol. and Plant Mol. Bio., Vol. 52: 29-66) 9.0 Alkaloids (Dayrit) 16 Overview Despite the wide variety and complex structures observed among the alkaloids, most of the compounds from this group are formed from only a handful of starting materials. The nitrogen and the initial carbon skeleton are derived from the following: A. aliphatic amino acids: lysine, glutamic acid, and ornithine; B. aromatic amino acids: phenylalanine, tyrosine, and tryptophan; and C. others: secologanin: terpene-derived C10 unit. 9.0 Alkaloids (Dayrit) 17 NH2 A. Aliphatic amino acids : lysine H2N CO2H NH2 ornithine H2N CO2H NH2 glutamic acid HO2C C. Others: CO2H CO2H B. Aromatic amino acids : phenlyalanine, R = H; CHO H H O tyrosine, R = OH H3COC NH2 R O secologanin CO2H tryptophan N H 9.0 Alkaloids (Dayrit) NH2 18 Overview The major reactions in alkaloid biosynthesis are common biosynthetic mechanisms: • Oxidation: epoxidation, aromatic hydroxylation, etc. • Oxidation: dehydrogenation (-2[H]); in the case of the alkaloids, this includes the conversion of amine imine. • Reduction: hydrogenation (+2[H]); in the case of the alkaloids, this includes the conversion of imine amine. • Phenolic dimerization by radical coupling. • Decarboxylation, in particular in the conversion of amino acid to amine. 9.0 Alkaloids (Dayrit) 19 Reactions which are particular to alkaloid biosynthesis. A. Mannich reaction : C-C aliphatic bond formation H C H C + O NH 2(CH 3) B. Aldehyde H3C C NH(CH3) H O - CH2 NH(CH3) H Amine via pyridoxal / pyridoxamine pyridoxamine R O CHO R pyridoxal CH2NH2 C. Bischler-Napieralski : isoquinoline biosynthesis + R NH2 C OR' -HOR' O phenylethylamine from phenylalanine N R + NH2 N tryptamine from tyrosine NH O R C OR' R -HOR' O N 9.0 Alkaloids (Dayrit) N O N N R 20 R Reactions which are particular to alkaloid biosynthesis. D. Pictet-Springler + NH2 R C H N O phenylethylamine from phenylalanine N R R + NH2 N tryptophan tryptamine from tyrosine R C H N O N N N R 9.0 Alkaloids (Dayrit) R 21 Aliphatic alkaloids The aliphatic alkaloids can be classified into three main types, depending on its biogenesis: • the amino acid ornithine • the amino acid lysine • the polyketide pathway with the nitrogen atom being introduced in a late step. 9.0 Alkaloids (Dayrit) 22 Aliphatic alkaloids from ornithine Glutamic is transformed into ornithine by addition of another CH2 unit. Therefore, in a way, glutamic acid is the original precursor and ornithine is the immediate precursor. Decarboxylation and loss of one nitrogen leads to formation of the pyrrolidine ring. There are three main types: pyrrolidine (monocyclic), tropane (bicyclic) and pyrrolizidine (fused). N N N N pyrrolidine tropane 9.0 Alkaloids (Dayrit) pyrrolizidine 23 Ornithine comes from glutamic acid. OHC HO2C H2N CO2H CO2H H2N N CO2H CO2H N glutamic acid H2N H2N CO2H H2N O CO2H ornithine 9.0 Alkaloids (Dayrit) 24 Pyrrolidine alkaloids from ornithine. Labeling studies show that although pyrrolidine itself is symmetric, the biosynthesis is regiospecific. This suggests that once ornithine is held by the enzyme, biosynthesis proceeds without release of any of the intermediates. * H2N 1. -CO2 2. pyridoxal # H2N CO2H * NH2 ornithine -CO2 H+ # # N * NH2 H+ N CH2 CH OH OH PO PO N H N CH3 CH3 [CH3] H2N H2N putrescine O O # * N _ H2C C # CH3 * +N CH3 CH3 NH CH2 hygrine OH PO N 9.0 Alkaloids (Dayrit) CH3 25 Tropane alkaloids from ornithine. Consistent with what has been observed, labeling shows that the biosynthesis is regiospecific. H3C O # H2N + N # # CO2H H2N H2C _ N CH3 ornithine O hygrine H3C N HO2C # CH2OH tropic acid H C atropine H3C N # H CH CH2OH O OH H3C N H3C N Ph O H3C Ph CH # N H3C benzoic acid CO2H O tropinone CO2H N O # OH Ph C H cocaine OH H O ecgonine 9.0 Alkaloids (Dayrit) tropine H 26 Pyrrolizidine alkaloids from ornithine. Pyrrolizidine alkaloids are common in the butterflies Senecio and Crotolaria species. NH2 H2N NH2 NH2 CHO CO2H CHO OHC -CO2 NH2 NH2 NH2 ornithine dimerize N 1. pyridoxal 2. +2[H] putrescine OH HO OH HO OH CHO O CH O CHO _ O O H3C H O N NH NH retronecine N retrorsine 9.0 Alkaloids (Dayrit) 27 Pyrrolizidine alkaloids are converted into aphrodisiac substances which the male butterflies store in its wing hair pencils. These compounds also protect the plants against feeding by mammals because these compounds are converted in the liver into toxic and carcinogenic compounds. O O OH HO O O OH HO [O] N + O_ retronecine-N-oxide N in mammalian liver N generalized pyrrolizidine in lepidoptera retronecine -2[H] [O] CHO HO H CHO OH HO N N danaidal N O HO hydroxydanaidal CH3 CHO sex phermones in lepidoptera N N + alkylating agent (carcinogenic) HO E-4-hydroxyhex-2-enal 9.0 Alkaloids (Dayrit) (bound to liver, toxic) danaidone 28 Polyhydroxylated cycloalkyl alkaloids found in the leaves, flowers and seeds of Ipomoea carnea (Convolvulaceae) cause natural intoxication of livestock by inhibiting key digestive enzymes. Alkaloids 1 and 2 are powerful inhibitors of lysosomal a-mannosidase; 3, 4, and 6 showed potent inhibitory activity toward rat lysosomal b-glucosidase; and alkaloid 5 was a moderate inhibitor of a- and b-mannosidases. (Haraguchi, et al., J. Agric. Food Chem. 2003, 51, 4995-5000.) HO OH H H OH OH N N Swainsonine ( 1) OH HO HO 2-epi-Lentiginosine ( 2) HN HN OH Calystegine B1 (3) HO HN OH OH HO HN OH OH OH OH Calystegine B2 (4) OH HO HO Calystegine B3 (5) 9.0 Alkaloids (Dayrit) OH OH OH Calystegine C1 ( 6) 29 Aliphatic alkaloids from lysine Lysine is modified following an analogous pathway to ornithine. There are many similarities between the ornithine-derived and lysine-derived alkaloids. The alkaloids produced are the 6-membered piperidine, homotropane and quinolizidine structures. N N N N piperidine homotropane 9.0 Alkaloids (Dayrit) quinolizidine 30 Alkaloids from lysine. 1-Piperidine and pellieterine are key intermediates to this group of alkaloids. O O O _ H2N H2N SCoA + N CO2H N H 1-piperidine lysine CSCoA O -CO2 H3C + N 1. [CH3] 2. [O] _ H2C + N CH3 O O N N O anahygrine (-)-pelletierine H3C O N + N H N -pelletierine O O N N N cernuine N anaferine N CH3 Quinolizidine alkaloids have the characteristic fused 6,6-bicyclic group and are derived from lysine. Lupinine is a dimeric metabolite while sparteine is trimeric. NH2 NH2 NH2 lysine O CH CO2H NH2 NH2 OHC NH2 N NH2 OHC cadaverine OH NH2 H N lupinine N N H sparteine 9.0 Alkaloids (Dayrit) 32 Aliphatic alkaloids from polyketides Some aliphatic alkaloids are derived from the polyketide pathway. The biogenesis of these alkaloids can be determined by studies using labeled acetyl CoA. * SCoA HO * * O O * * * O * * H2N * * * * N -coniceine * O * * * * N OH conhydrine 9.0 Alkaloids (Dayrit) * * * * N coniine 33 Polyketide-derived piperidine alkaloids. Some alkaloids having the piperidine-type structure are not derived from lysine. * O * SCoA * * * O O * CO2H -CO 2 * * * N penidine The European ladybug ( Coccinella septempunctata ) produces this tricylic defense substance. H O O O O O N -CO 2 H HO2C H O a heptaketide coccinelline 9.0 Alkaloids (Dayrit) 34 Alkaloids from phenylalanine and tyrosine The aromatic alkaloids derived from phenylalanine and tyrosine form a diverse and often structurally complex group of metabolites. By tradition, these alkaloids are identified according to plant family, of which the best known are: Papaveraceae, Morphinan, Erythria, Berberidaceae, Amaryllidaceae. Structurally and biosynthetically, there are six main groupings: 1. Phenylethylamines 2. Phenylethylamine + alkyl aldehyde or ketone 3. Phenylethylamine + benzaldehyde (C6-C1) 4. Phenylethylamine + C6-C2 5. Phenylethylamine + C6-C3 6. Phenylethylamine + secologanin 9.0 Alkaloids (Dayrit) 35 Alkaloids from phenylalanine/tyrosine. Structurally, there are six main types. NH2 1. Simple phenylethylamines. 2. Phenylethylamine + alkyl aldehyde or ketone. + R CHO NH NH2 R H N 3. Phenylethylamine + benzaldehyde (Ar-C 1). OHC NH2 + 4. Phenylethylamine + Ar-C 2. NH2 NH OHC 9.0 Alkaloids (Dayrit) 36 Alkaloids from phenylalanine/tyrosine. Structurally, there are six main types. 5. Phenylethylamine + Ar-C 3. NH2 NH OHC 6. Phenylethylamine + secologanin. NH2 NH OHC O-Glc O- Glc O CH3 OC O 9.0 Alkaloids (Dayrit) O CH3 OC O 37 NH2 CO2H HO N(CH3)2 NH2 -CO2 HO HO hordenine tyramine tyrosine Hordenine is produced by the barley plant. It is released in the roots and acts to kill competing plants, in particular weeds. It is an allelopathic agent. [O] HO NH2 CO2H HO DOPA -CO2 HO 1. [O] 2. [CH3] NH2 HO HO NH2 HO dopamine Mescaline is the hallucinogenic compound in the peyote cactus (Lophophora williamsii). It is produced together with a number of other phenylethylamines. OCH3 mescaline [O] OH HO NH2 HO norepinephrine [CH3] OH HO NH(CH3) Epinephrine is also a cactus alkaloid. It is also a human hormone secreted by the adrenal medulla which acts to increase the heart rate, blood pressure and carbohydrate metabolism. It is also known as adrenalin. 9.0 Alkaloids (Dayrit) HO epinephrine Alkaloids from phenylalanine/ tyrosine: Simple phenylethylamines. Biosynthesis of this group involves simply loss of the carboxylic acid carbon. Some important members of this group are the hallucinogenic compound mescaline and the drug epinephrine. 38 NH2 CO 2H leucine O HO 1. NH2 HO CH3O CO 2H 2. [CH3] Lophocerin was isolated from the cactus Lophocerus schotti. NHCH3 HO dopamine lophocerine CH3O OHC CO 2H glyoxylic acid NH2 CH3O CH3O CH3O -CO2 NH2 CH3O OH OH O NH 2 HO 2C H 3C C CO 2 H CO 2H pyruvic acid NH2 CH3O CO2H OH peyoxylic acid anhalamine glutamic acid CH3O CH3O O HO 2C -CO2 NH2 CO 2H CH3O OH H3C CO2H CH3O peyoruvic acid N CH3O NH2 CH3O OH pellotine CH3 Alkaloids from phenylalanine/ tyrosine: Condensation of phenylethylamine with alkyl aldehydes or ketones. This group of alkaloids is formed via a PictetSpringler or Bischler-Napieralski condensation. The alkaloids shown here are found in the hallucinogenic peyote cactus plant. O OH peyoglutan 9.0 Alkaloids (Dayrit) 39 Alkaloids from phenylalanine / tyrosine + Ar-C1. Phenylethylamine couples with Ar-C1 (benzaldehyde) via a Pictet-Springler condensation. This is followed by oxidation of the phenol. HO CO2H NH2 HO -CO 2 NH2 HO HO NH CHO CH3O CH3O HO o-methylnorbelladine [O] O O O NH . O . NH CH3O CH3O . . . O 9.0 Alkaloids (Dayrit) NH CH3O O 40 O O O . . . NH . NH . CH3O CH3O CH3O NH . O O O o-p O O O CH3O . . O . . NH O NH CH3O p-p O CH3O NH HO CH3O 1. p-o 2. [CH3] OH NH O Alkaloids from phenylalanine / tyrosine + Ar-C1. The two oxidized rings can couple via different folding conformations leading to parapara, para-ortho or ortho-para coupling. HO OH O O O O NCH3 CH3O lycorine O O N 9.0 Alkaloids oxocrine galanthamine N (Dayrit) 41 HO CO2H -CO 2 NH2 HO NH2 HO HO NH CHO CH3O CH3O HO o-methylnorbelladine [O] O O O . . . NH . NH . CH3O CH3O CH3O NH . O O O o-p O O O CH3O . . O . . NH O NH CH3O p-p O CH3O NH HO CH3O 1. p-o 2. [CH3] OH NH O HO Alkaloids from phenylalanine / tyrosine + Ar-C1. (overview) Coupling of the radical intermediates in different folding conformations leads to parapara, para-ortho and orthopara couplings. This is a theme that is repeated for other alkaloids with similar structural characteristics. The alkaloid families that comprise this group include the Amaryllidaceae and Mesembrine species. OH O O O O NCH3 lycorine O N O CH3O oxocrine galanthamine N 9.0 Alkaloids (Dayrit) 42 Alkaloids from phenylalanine / tyrosine + Ar-C2. Condensation of phenylethylamine with an Ar-C2 group, such as phenylpyruvic acid, yields the benzyltetrahydro isoquinoline structure. These alkaloids are characteristic of the Papaveraceae. Reticuline is a key intermediate of this group. HO HO CO2H -CO2 HO NH2 HO N NH2 HO DOPA HO CO2H pyridoxal pyrophosphate HO -CO2 OH O HO 1,2-dehydrolaudanosoline 1. +2[H] (2. 2x [CH3]) RO OH 1. -2[H] 2. 4x [CH3] CH3O NR N HO CH3O H OH (S)-norlaudanosoline, R = H (S)-reticuline, R=CH3 9.0 Alkaloids (Dayrit) OR OCH3 papaverine 43 OCH3 Alkaloids from phenylalanine / tyrosine + Ar-C2. The Aprophine alkaloids are produced by oxidation of reticuline. Various isomeric radical intermediates are formed. CH3O NCH3 HO H HO (S)-reticuline CH3O [O] CH3O CH3O O O . . NCH3 H O CH3O A NCH3 . H O O A NCH3 H . H O O CH3O C NCH3 O H . . C B . . NCH3 A H O A O B NCH3 CH3O CH3O . CH3O CH3O . . NCH3 C CH3O CH3O B O C CH3O O . B O . CH3O CH3O . CH3O O H Alkaloids from phenylalanine / tyrosine + Ar-C2. Four regiochemical couplings are observed: ortho-ortho, ortho-para, para-ortho, and para-para. CH 3O CH 3O O O NCH 3 . . O H CH3O NCH3 . . O CH 3O C . . O H C B . . NCH3 A A O o-o O o-p CH 3O O p-p CH 3O NCH 3 H H CH 3O p-o CH 3O O B NCH 3 CH3O CH 3O CH 3O H OCH 3 NCH3 O H O CH 3O O H NCH 3 H CH 3O CH 3O CH 3O O NCH 3 H O O 1. 2[H] 2. -H2O CH 3O NCH 3 O H NCH 3 O CH 3O O sebiferine CH3O H CH3O O CH 3O NCH3 CH 3O bulbocarpine H OH isoboldine CH3O thebaine (to the morphine alkaloids) 45 Alkaloids from phenylalanine / tyrosine + Ar-C2. The Aprophine alkaloids are produced by radical coupling of the benzylisoquinoline system of reticuline. O CH3O CH3O O O NCH3 . . O H CH3O CH3O NCH3 . . H C C . . . . O B A CH3O CH3O O O p-p OCH3 CH3O CH3O NCH3 O H H H O p-o CH3O NCH3 A CH3O o-p o-o NCH3 H CH3O O CH3O B NCH3 O H O NCH3 NCH3 H CH3O CH3O H CH3O O CH3O O O NCH3 O H O 1. 2[H] 2. -H2O CH3O NCH3 O sebiferine CH3O H CH3O O CH3O CH3O bulbocarpine NCH3 H OH isoboldine CH3O thebaine (to the morphine alkaloids) Schematic presentation of the biosynthesis of codeine, laudanine, and (S)-scoulerine from (S)norcoclaurine in the opium poppy. The cellular localizations of the enzymes indicated have been determined experimentally. (Jorgensen et al., Curr Opinion in Plant Biol 2005, 8:280–291) 9.0 Alkaloids (Dayrit) 47 Alkaloids from phenylalanine/tyrosine + Ar-C2. The morphine alkaloids are produced from thebaine. Note that the level of methylation decreases towards the end of the biosynthetic sequence from thebaine codeine morphine. CH3O CH3O O HO O NCH3 H CH3O 1. -[CH3] 2. +2[H] H O H H HO thebaine NCH3 -[CH3] NCH3 H HO codeine 9.0 Alkaloids (Dayrit) morphine 48 CH3O Alkaloids from phenylalanine / tyrosine + Ar-C2. Coupling using the N-methyl group. CH3O N-CH3 HO -H- H N + HO OH CH2 H O-H (S)-reticuline OCH3 OCH3 O CH3O N N O HO H H OCH3 O [O] (S)-canadine OH (S)-scoulerine OCH3 N+ OCH3 O OCH3 O berberine OCH3 N O H O (S)-stylopine O [CH3] O O N +N CH3 CH3 O O H O O O protopine 9.0 Alkaloids (Dayrit) O O 49 CH 3O HO HO -C O2 CO 2H NH 2 HO HO CH 3O NH 2 HO NC H3 HO CO 2H 1. pyrido xal pyropho sphate 2. +2[H] HO 3. -H2O DO PA autum naline (cf. re ticuline) CH 3O OH [O ] CH 3O CH 3O NC H3 HO NC H3 o-p O . . CH 3O CH 3O CH 3O CH 3O OH Alkaloids from phenylalanine / tyrosine + Ar-C3. This group is homologous to the benzyltetrahydroisoquinolines (dopamine + C2). The biosynthetic steps are assumed to be similar. O multif loramine CH 3O O CH 3 O CH 3 . CH 3O CH 3O A CH 3O O p-o NC H3 CH 3O B C NC H3 H CH 3O H . . A H o-m ethyland ro cymb ine . CH 3O CH 3O O NC H3 O C [CH3 ] B O O 9.0 Alkaloids (Dayrit) 50 Alkaloids from phenylalanine / tyrosine + secologanin. Secologanin is an iridoid belonging to the monoterpene group. Condensation of phenylethylamine with secologanin leads to a group of mixed metabolites. HO OHC NH2 HO dopamine HO - O Glc + NH O HO H3COC O O-Glc secologanin O H3COC O -Glc HO NH HO O-H O H3COC O 9.0 Alkaloids (Dayrit) 51 HO HO HO H+ 1 NH CO2H HO NH NH HO HO O 1 H O O-H O 2 H O H3COC H O H3COC O H O H+ H 2 HO HO N HO O NH HO OH H N+ CHO HO H O HO O H3COC O H H O 1. 2. 3. 4. HO N O H3COC +Glc O HO CH3O CH3O H H+ -CO 2 2[CH 3] [H] CH3O HO N alangiside Alkaloids from phenylalanine / tyrosine + secologanin. NH2 HO N CH3O O emetine O-Glc H proemetine H OH O HN 9.0 Alkaloids (Dayrit) OH H 52 Alkaloids from tryptophan: the indole alkaloids • The indole alkaloids are derived from tryptophan and are found in both plants and microorganisms. They comprise the single largest group of alkaloids, accounting for almost onefourth of all alkaloids isolated. Many of the members of this group are biologically active and some possess very important medicinal properties. Among the best known sources are: Catharanthus, Curare, Rauwolfia, and Vinca plant species and the ergot fungi. • The indole alkaloids can be classified as follows: 1. Simple indole alkylamines 2. Simple b-carbolines 3. Tryptophan + C5 4. Tryptamine + secologanin 9.0 Alkaloids (Dayrit) 53 Indole alkaloids. Structural groups.. 1. Simple indole alkylamines. N N 2. Simple b-carbolines: indole + aldehyde (Pictet-Springer condensation) N + N N N OHC R may be saturated or aromatic R 9.0 Alkaloids (Dayrit) 54 Indole alkaloids. Structural groups.. 3. Tryptophan + C 5 (ergolines). + OPP CO2H CO2H NH2 NH2 N N 4. Tryptamine + secologanine. (Pictet-Springer condensation) NH2 N N CHO N O-Glc O-Glc + O CH3OC O CH3OC O O 9.0 Alkaloids (Dayrit) 55 Simple indole alkaloids. Decarboxylation of tryptophan yields serotonin, a neurotransmitter; methylation yields bufotenin, a hallucinogenic compound isolated from toadstools. CO2H HO NH2 NH2 N H 1. -CO2 2. [O] N H 5-hydroxytryptamine (serotonin) [CH3] HO N(CH3)2 N H bufotenin CH3 HO N CH3 eseroline N CH3 9.0 Alkaloids (Dayrit) 56 Simple indole alkaloids. Bishler-Napieralski condensation of tryptamine with simple alkyl groups yields the b-carbolines. The harmanes are CNS stimulants. N NH O N N C H3C CO2H H3C -CO2 N N CO2H harmane 9.0 Alkaloids (Dayrit) CH3 57 Ergot alkaloids. Condensation with C5 DMAPP. This group of metabolites is produced by the fungus Claviceps purpurea and includes the hallucinogen lysergic acid. + OPP O CO2H CO2H _ CO2H [O] NH2 NH2 NH2 N N N H H H OPP HO2C NCH3 HO HO2C NH2 HO 1. -CO2 1. [CH3] 2. [O] N H NH2 2. [O], +OPP N H N H lysergic acid 9.0 Alkaloids (Dayrit) 58 N N N N N N O O Vincosan (D-type) Coryanthean (C-type) N N N N N N Reserpine Valleseachotaman (V-type) N N N N N Strychnan (S-type) Eburnan (E-type) N Aspidospermatan (A-type) Plumeran (P-type) N N Ibogan (I-type) Indole alkaloids: tryptophan + secologanin. More than 1,100 compounds from this group of mixed metabolites have been identified. They occur predominantly in Loganiaciae, Apocynaceae, and Rubiaceae. This figure gives the major skeletal types. NH2 N NH NH N N + O-Glc CHO O-Glc H O-Glc + O O CH3OC H O CH3OC O CH3OC O strictosidine O vincoside H+ -Glc NH NH NH N N N CHO O-H CHO O OH OH CH3OC CH3OC CH3OC O O O N N +2[H] H N+ N N N H H O CH3OC O ajmalicine H O CH3OC O catharanthine OH CH3OC O Indole alkaloids: tryptophan + secologanin. The Vincosan alkaloids yield straightforward incorporation of secologanin. • Loss of glucose enables more extensive structural changes to occur. The glycoside is hypothesized to act as a protecting group. • Many alkaloids are formed from strictosidine. NH NH N N O-Glc O CH3OC O 1. -Glc 2. H+ 3. double bond isomerization 4. bond rotation CHO O CH3OC O strictosidine +N H +N N N N N H H O CH3OC CH3OC O O O OH N CH3OC O H H H Indole alkaloids: tryptophan + secologanin. Loss of glucose initiates the chemical transformation. Reserpine is a tranquilizer and sedative isolated from the roots of Rauwolfia serpentina. N N N H H H O H H CH3OC CH3OC O OH O a-yohimbine reserpine OCH3 O OCH3 OCH3 9.0 Alkaloids (Dayrit) OCH3 61 N NH N N H N N O OH CH3OC CH3OC H H+ O-Glc CH3OC O O O geissoschizine strictosidine CHO N N N H N+ N H CH3OC HO O 1. [O] 2. -CH2O N H CH3OC CHO O [O] CH3COS-CoA N N N H N CoAS HO O O O O strychnine 9.0 Alkaloids (Dayrit) CH3OC O _ CHO Indole alkaloids: tryptophan + secologanin. Strychnine (from the seeds of Strychnos nux-vomica) was used in medicine as a CNS stimulant. At higher doses, it was used as poison for humans and rodents. 62 NH N N H N H O-Glc O CH3OC CHO O strictosidine [O] corynantheal O-H X CHO CHO N N N N H N 1. -H2O 2. +2[H] 3. "X" H N H HO H O H H2O X Indole alkaloids: tryptophan + secologanin. The cinchona alkaloids (from the bark of Cinchona spp.) involve extensive rearrangement.Quin ine was used as an antimalarial drug. CHO N NH2 O O NH2 H O N X N 1. -H2O 2. -"HX" CHO N cinchonidinone H N HO R quinidine, R=OCH3 cinchonine, R=H HO H R N N quinine, R=OCH3 cinchonidine, R=H 9.0 Alkaloids (Dayrit) N 63 Alkaloids from other pathways Other groups of alkaloids arise from various pathways. A number of them are metabolites from other biogenetic groups, but are classified as alkaloids simply because they have an amine functionality. This mixed group of alkaloids includes: • the quinoline alkaloids (from anthranilic acid, shikimates) • terpene alkaloids • nicotine alkaloids • xanthine alkaloids. 9.0 Alkaloids (Dayrit) 64 CO2H shikimate NH2 O anthranilic acid O O O SCoA SCoA O O O O SCoA O NH2 O O SCoA O SCoA CO2H O O O NH2 O O 2. [CH3] 3. [O] 4. [CH3] N H N O NH2 OH OH H 1. PPO OH N 1. [O] 2. [CH3] OCH3 O OH OCH3 CH3O N Quinoline alkaloids. Except for the cinchona alkaloids, the quinoline alkaloids are mixed metabolites being derived from anthranilic acid, which belongs to the shikimate group, and polyketides. O OCH3 skimmianine N CH3 arborinine OCH3 9.0 Alkaloids (Dayrit) 65 The terpene alkaloids. The sequence of addition of nitrogen into the terpene is suggested to be: R-CH2-OH R-CHO R-CH2-NH2 (where R is a terpene metabolite). A. Monoterpene alkaloids. OHOPP 1. [O] 2. OPP geraniol O PP OH OH [O] OH CHO pyridoxamine NH2 OPP H N N CH3 dehydroskythanthine 9.0 Alkaloids (Dayrit) actidine 66 The terpene alkaloids. Steroidal alkaloids are formed from completed steroids. Solasidine and tomatidine occur in potatoes and tomatoes, respectively. B. Steroidal alkaloids. HO cholesterol O O CHO CHO O O NH2 NH2 N CH3 N N CH3 25S 25R N CH3 CH3 O O solasidine HO HO 9.0 Alkaloids (Dayrit) tomatidine 67 Nicotine alkaloids Tobacco (Nicotiana tabacum) is another plant from which a large commercial sector has formed. It is a practice that originated from the American Indians. Nicotiana comes from the name of Jean Nicot, a French diplomat who probably introduced the habit to Europe; tabacum comes from the Indian name for the pipe that was used to smoke it. Nicotine, the chief constituent of N. tabacum, is formed from nicotinic acid. CO2H N CH3 N N nicotinic acid (-) nicotine 9.0 Alkaloids (Dayrit) 68 Nicotinic acid. In plants, nicotinic acid is biosynthesized from tryptophan. A. Biosynthesis from tryptophan (in plants) [O], Baeyer-Villiger CO2H CO2H NH2 [O] O N H N tryptophan NH2 CO2H O -CH 2O NH2 NH2 CH O [O] CO2H CO2H [O] CO2H CO2H [O] OHC NH2 HO2C NH2 O O OH cis NH2 NH2 OH OH 3-hydroxyanthranilic acid trans CO2H CO2H CO2H OHC H2N CO2H N CO2H quinolinic acid N nicotinic acid 9.0 Alkaloids (Dayrit) 69 Nicotinic acid. In bacteria, nicotinic acid is biosynthesized from glyceraldehyde + aspartic acid. B. Biosynthesis from glyceraldehyde + aspartic acid (C 3 + C4) (in bacteria) OPP HO _ CO2H - CO2H CO2H CO2 CH O HN 2 - CO2 N CO2H N CO2H quinolinic acid 9.0 Alkaloids (Dayrit) N nicotinic acid 70 Nicotinic acid. Mimosine, which resembles phenylalanine, is a toxin found in grass which is used as animal feed. A. Various nicotine alkaloids O O C-NH2 C-OGlc N nicotinamide + N Glc O OH OH OH Mimosine, a -pyridone which is found in Mimosa species, is toxic to animals. Note that it is a a-amino acid and is believed to mimic phenylalanine, tyrosine or DOPA. N CO CO22H H buchanine NH NH22 mimosine 9.0 Alkaloids (Dayrit) 71 Nicotinic acid. Biosynthesis of nicotine. Nicotine is a ganglionic cholinergic-receptor agonist. Chronic ingestion of nicotine often results in psychological addiction and physical dependence. B. Biosynthesis of nicotine (Nicotiana alkaloids). CO2H H2N H2N H2N CO2H CO2H ornithine N lysine H2N -CO 2 + N CH3 N N CH3 N N N N (-) nicotine anabasine N N N anatabine 9.0 Alkaloids (Dayrit) 72 Xanthine alkaloids are important components of a number of culturally, historically and commercially important plants, in particular coffee, cola (kolanut), tea and cacao (chocolate). The active constituents are methyl xanthines, the best known of which are: caffeine, which occurs in coffee (Coffea arabica); theophylline, which is found in tea (Camellia sinensis); and theobromine, which is found in cacao (Theobroma cacao). Note that theophylline and theobromine are isomers. O H N N N N N Purine O H3C O N N CH3 Caffeine H N O N N Xanthine O O CH3 N H3C N O H N N N N CH3 Theophylline 9.0 Alkaloids (Dayrit) N H N O CH3 N N CH3 Theobromine 73 The methylxanthines (caffeine, theophylline and theobromine) are CNS stimulants and smooth muscle relaxants. Research into their physiological mechanisms are continuing research topics. CO 2 aspartic acid H33C C H glycine, NH2CH2CO2H O C N1 6 5 C CH3 C1 fragments N 7 8 CH C2 3 4 C 9 N O O N C1 fragments CH3 Caffeine amide N of glutamine The biogenetic origin of xanthine is complex and arises from various primary metabolites. Carbons 2 and 8 come from an active 1-carbon fragment (e.g, formate, methyl methionine, etc.); carbon 6 comes form CO2; and carbon atoms 4 and 5 and nitrogen 7 come from glycine. The nitrogen atom at 1 comes from aspartic acid, while those at 3 and 9 9.0 Alkaloids (Dayrit) 74 come from the amide nitrogen of glutamine. Summary • Structurally, the alkaloids are a very diverse group; the only unifying characteristic is the presence of an amine. • The origin of the carbons in alkaloids include the aliphatic amino acids (ornithine and lysine), aromatic amino acids (phenylalanine, tyrosine and tryptophan, which arises from shikimic acid via phenylpropanoids), anthranilic acid (from shikimic acid), polyketides, and terpenes. 9.0 Alkaloids (Dayrit) 75 Summary The alkaloids are divided into characteristic structures, which are also usually associated with specific plants or organisms. Among the best known groups of alkaloids are: Tropane alkaloids (e.g., Atropa) Pyrrolizidine alkaloids Phenylethylamines: (e.g., Ephedra) Phenylalanine + C6-C2: (e.g., Aprophine, Papaver and Erythrina) Tryptophan + DMAPP: (e.g., ergot alkaloids) Tryptophan + secologanin: (Vinca, Catharanthus, Strychnos, Cinchona) Steroidal alkaloids Nicotinic acid: (Nicotiana) Xanthine alkaloids: (Coffea, Theobroma) 9.0 AlkaloidsCamelia, (Dayrit) 76 Overview of alkaloid biosynthesis. The biogenetic location of the xanthines is diverse and not included here. glucose aliphatic amino acids lysine, ornithine shikimate anthranilic acid phenylalanine, tyrosine, tryptophan polyketide piperidine mevalonic acid terpene alkaloids: monoterpene, steroidal C5-OPP, secologanin (to aromatic alkaloids) aliphatic alkaloids: pyrrolidine, tropane, pyrrolizidine; piperidine, quinolizidine quinoline aromatic alkaloids: phenylethylamine, isoquinoline, betalin; indole, carboline, quinoline, nicotinic acid 9.0 Alkaloids (Dayrit) 77