* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Amines and Amides
Bottromycin wikipedia , lookup
Genetic code wikipedia , lookup
Peptide synthesis wikipedia , lookup
Expanded genetic code wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Metalloprotein wikipedia , lookup
Nitrogen cycle wikipedia , lookup
Biosynthesis wikipedia , lookup
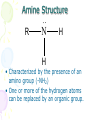
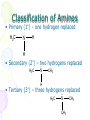
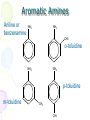
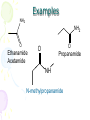
Amines and Amides Chapter 16 Nitrogen • Fourth most common atom in living systems. • Important component of the structure of nucleic acids, DNA, and RNA • Essential to the structure and function of proteins – enzymes and antibodies Amine Structure R N H H • Characterized by the presence of an amino group (-NH2) • One or more of the hydrogen atoms can be replaced by an organic group. Amines • Organic derivatives of ammonia and, like ammonia, they are basic. H N H H R N H H • Amines are classified according to the number of alkyl groups attached to the nitrogen. Classification of Amines • Primary (1°) – one hydrogen replaced H3C N H H • Secondary (2°) – two hydrogens replaced H3C N CH3 H • Tertiary (3°) – three hydrogens replaced H3C N CH3 CH3 Properties of Amines • Because nitrogen is more electronegative than hydrogen, the N-H bond is polar. • Hydrogen bonding can occur between amines and water. • Primary and secondary amines have higher boiling points than tertiary amines because tertiary amines cannot form hydrogen bonds. • Small amines (six or fewer carbons) are soluble in water. Naming Amines - IUPAC • Least common system used • Parent chain is longest continuous chain to which the amine group is attached. • Use a number to indicate the position of the alkane. • Add the prefix “amino” to the name of the alkane. • If a substituent is present on the nitrogen, it is designated by the prefix N-alkyl. Naming Amines - IUPAC CH3NH2 CH3CH2NH2 CH3NHCH3 CH3NHCH2CH3 aminomethane aminoethane N-methylaminomethane N-methylaminoethane CH3 N,N-dimethylaminomethane CH3NCH3 Naming Amines – CA system • CA system – Chemical Abstracts system • The final –e of the parent name is dropped and the suffix –amine is added. • For secondary and tertiary amines, the prefix N-alkyl is added to the parent compound. Naming Amines – CA system CH3NH2 CH3CH2NH2 methanamine ethanamine CH3NHCH2CH3 CH3NHCH3 N-methylmethanamine N-methylethanamine CH3 N,N-dimethylmethanamine CH3NCH3 Aromatic Amines Aniline or benzenamine NH2 NH2 CH3 o-toluidine NH2 NH2 p-toluidine m-toluidine CH3 CH3 Other Common Name Amines CH3NH2 methylamine CH3NHCH3 dimethylamine CH3 CH3NCH3 CH3CH2NH2 ethylamine CH3NHCH2CH3 ethylmethylamine trimethylamine Amides • Products formed in a reaction between a carboxylic acid and an amine. • Contains two portions: the carbonyl group from a carboxylic acid and the amino group from the amine O R C NH2 Amide Bond Properties of Amides • Most are solids at room temperature • Very high boiling points – hydrogen bonding between N-H bond of one amide and the C=O group of the second amide • Simpler ones are quite soluble in water • Unlike amines, amides are NOT bases Naming Amides - IUPAC • Replace the –oic acid from the carboxylic acid with the ending – amide. • Substituents on the nitrogen are placed as prefixes and are indicated by N- followed by the name of the substituent. Examples NH2 NH2 O Ethanamide Acetamide O O Propanamide NH N-methylpropanamide