* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Structure of HIV-1 gp120 with gp41-interactive
Citric acid cycle wikipedia , lookup
Butyric acid wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Genomic library wikipedia , lookup
Peptide synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Proteolysis wikipedia , lookup
Homology modeling wikipedia , lookup
Catalytic triad wikipedia , lookup
Genetic code wikipedia , lookup
Point mutation wikipedia , lookup
Biochemistry wikipedia , lookup
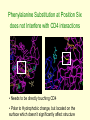
Mutated Amino Acid Sequences in V3 Domain of gp120 Show No Significant Correlation to Altered Folding and Function Bobak Seddighzadeh Alex George Loyola Marymount University March 23, 2010 Outline 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Levels of protein organization Amino acid characteristics and classifications Determinants of Tertiary and Quaternary Structures DNA mutations can affect protein function What led us to our question Multiple Sequence alignment shows key insight on unconserved regions of V3 domain Analysis of the amino acid substitutions found in the multiple sequence alignment In depth structural analysis of key amino acid substitutions Scan prosite indicates the function of amino acids at unconserved regions of V3 domain Correlation and conclusion of mutations on structure and function in V3 domain Levels of Protein Organization Affect Overall Function • Primary Structure – The number and sequence of amino acids • Secondary Structure – Alpha Helices – Beta-pleated Sheets • Tertiary Structure – 3-D shape of structure • Quaternary Structure – Intra-protein interactions QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Amino Acid Characteristics Determine Molecular Interactions Classified into Four Types based on R-Group: 1. Uncharged Polar • 2. Nonpolar • 3. Hydrophobic Acidic • 4. Hydrophilic Positive charge Basic • Negative charge * Proline and Glycine are unique QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Amino Acid Side Chains Play a Large Role in Tertiary and Quaternary Structure 1. 2. 3. 4. 5. Covalent disulfide bonds Electrostatic Interactions Hydrogen bonds Van der Waals forces Hydrophobic Side Chains Mutations in DNA Sequence Can Affect Tertiary Protein Structure • Central Dogma: DNARNAProtein Example: • Sickle Cell Anemia – Single point mutation changes Glutamic acid (hydrophilic) to Valine (hydrophobic) – Results in dysfunctional folding of Hemoglobin A (Tertiary) QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Determining the Effects of Amino Acid Alterations in Conserved Sequences • Analysis of sequences from Markham et. al study confirmed the presence of highly conserved regions in the V3 loop of gp120 • Kwong et. al study hypothesize the conserved regions were necessary for viral cell entry • Our question: Will amino acid mutations in conserved regions of amino acids alter/disrupt the function of the protein Rapid and non Progressors Sequences were Chosen According to High Diversity and Divergence • Rapid Progressors: – Subject 4 • Visit 4 - clones 4,1 • Visit 3 - clones 16,2 • Visit 2 - clones 13, 5 – Subject 10 • Visit 6 - clones 7,4 • Visit 5 - clones 10,3 • Visit 4 - clones 8,5 – Subject 11 • Visit 4 - clones 8,6 • Visit 3 - clones 5,2 • Visit 2 - clones 6,1 • Non Progressors: – Subject 13 • Visit 2 - clones 1,2 • Visit 3 - clones 3,6 • Visit 5 - clones 3,6 – Subject 12 • Visit 5 - clones 6,5 • Visit 4 - clones 2,1 • Visit 3 - clones 3,1 – Subject 2 • Visit 4 - clones 6,5 • Visit 3 - clones 5,4 • Visit 1 - clones 5,1 Multiple Sequence Alignment for Subjects 2,4,10,11,12,13 Fully conserved Strongly conserved Weakly Conserved Figure: Highlights conserved, unconserved, and similar amino acid positions in the V3 domain of gp120 amongst these subjects Gp120 in complex with CD4 and two antibodies with the V3 region highlighted gp120 Human antibody CD4 Receptor V3 loop is located on the periphery of the protein More Non-conservative than Conservative Amino Acid Substitutions were Found Between Clones Position 6 10 10 10 10 15 15 20 23 23 23 23 23 23 25 25 39 39 39 43 71 88 88 88 Initial amino acid Final amino acid Serine S13 V5-3 Threonine S2 V3-4 Threonine S11 V4-8,6 Threonine S11 V3-5,2 Threonine S11 V2-6,1 S10 V5-10,3 V6-7,4 Isoleucine Isoleucine S13 V5-6 Leucine S2 V4-3 Serine S10 V5-10 Serine S10 V4-6 Serine S10 V6-7 Serine S12 V3-3,1 Serine S12 V4-2,1 Serine S12 V5-6,5 Glutamic acid S4 V3-16 Glutamic acid S11 V4-8,6, V3-5,2, V2-6,1 Serine S10 V5-10, V4-6,8, V6-4 Serine S10 V5-3 V3-2 Serine S4 V3-16, V2-13, V4-1, Glycine S2 V4-8 Aspargine S12 V5-6 Glutamine S13 V2-1,2 Glutamine S13 V3-3.6 Glutamine S13 V5-3,6 Phenylalanine Methionine Serine Serine Serine Threonine Threonine Proline Alanaine Alanaine Alanaine Threonine Threonine Threonine Glycine Valine Arginine Lysine Lysine Arginine Aspartate Histadine Histadine Histadine Subject Characteristic Change Polar to Hydrophobic Polar to Hydrophobic Polar to Polar Polar to Polar Polar to Polar Polar to Hydrophobic Polar to Hydrophobic Hydrophobic to uncharged rigid ring structure Polar to Hydrophobic Polar to Hydrophobic Polar to Hydrophobic Polar to Polar Polar to Polar Polar to Polar Negative charge to Uncharged Negative charge to Hydrophobic Polar to Positive charge Polar to Positive charge Polar to Positive charge Uncharged to Positive charge Polar to Negative charge Polar to positive charge Polar to positive charge Polar to positive charge Phenylalanine Substitution at Position Six does not Interfere with CD4 interactions • Needs to be directly touching CD4 • Polar to Hydrophobic change, but located on the surface which doesn’t significantly affect structure Threonine to Serine Substitution at Position Ten has minimal affect on Structure • Serine and Threonine both have hydroxyl's on their side chain making them structurally similar • The residue substitutions on the surface don’t affect structure significantly At position twenty the substitution from Leucine to Proline May Affects the -sheet • The amino acid sequence is buried in the peptide • The direction and nature of the Beta turn can be altered Amino Acid Substitution Serine to Alanine at Position Twenty-three has Minimal Affect • Serine and Alanine are very similar in size • The residue is located on the surface of the protien -sheet Is Unaffected by Amino Acid Substitution to Glycine at Position Twenty-five • B-pleated Sheets are very forgiving • Glycine is more likely to affect Alpha helices • The residue is located on the surface of the protien Alpha Helices May be Affected by Substituting Glycine to Arginine at Position Forty-three • Lysine to Arginine substitution is small uncharged to bulky positive charge • The residue is located on the surface of the protein structure Post-Transcriptional modifications likely occur at common amino acid sequences in Kwong’s sequence • N-Glycosylation – Typical Sequence = Asn-X-Ser or Asn-X-Thr – Important in folding and cell-cell interaction • Phosphorylation – Increases energy so that the protein can undergo subsequent reactions spontaneously – Charged amino acids at the N-terminus affect phosphorylation rate – Position 25 mutation (Glutamic acid Glycine/Valine) is at the C-terminus of the acceptor site, thus not affecting function Mutations in un-conserved regions of the V3 Domain do not greatly affect function of gp120 • The structure of the V3 domain remains relatively unaffected by un-conserved mutations • The location of the V3 domain may serve as a defense to mutational changes • Analysis of the other domains of gp120 may better suit our investigation Conserved Elements Between gp120 and gp41 May Play Large Role in Viral Entry • Conformational changes in gp120 affect drug and antibody neutralization • The association between gp120 and gp41 plays a role in determining • Defined elements between gp120 and gp41 provides conformational diversity necessary for viral entry • Pancera et. al References Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WAStructure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody Nature v393, p.648-659 Markham RB, Wang WC, Weisstein AE, Wang Z, Munoz A, Templeton A, Margolick J, Vlahov D, Quinn T, Farzadegan H, and Yu XF. Patterns of HIV-1 evolution in individuals with differing rates of CD4 T cell decline. Proc Natl Acad Sci U S A 1998 Oct 13; 95(21) 12568-73. pmid:9770526. Pancera M, Majeed S, Ban YE, Chen L, Huang CC, Kong L, Kwon YD, Stuckey J, Zhou T, Robinson JE, Schief WR, Sodroski J, Wyatt R, Kwong PD Structure of HIV-1 gp120 with gp41-interactive region reveals layered envelope architecture and basis of conformational mobility Proc. Natl. Acad. Sci. U. S. A. v107, p.1166-1171