* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The structure of cellulose
Survey
Document related concepts
Transcript
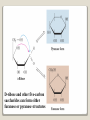
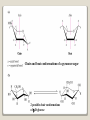
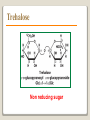
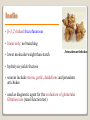
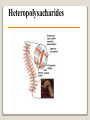
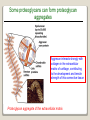
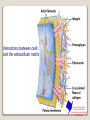
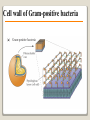
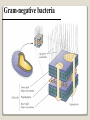
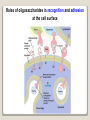
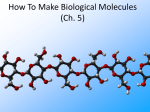
1 References • Lubert Stryer - Biochemistry • Lehninger- Principles of Biochemistry • Harper's -Illustrated Biochemistry • Text Book of Biochemistry with clinical correlation, T M. Devlin انتشارات، دانشگاه علوم پزشکی تهران، هیئت مولفان،•بیوشیمی پزشکی دو جلد،آییژ Carbohydrates General characteristics Compounds composed of C, H, and O (CH2O)n when n = 5 then C5H10O5 Not all carbohydrates have this empirical formula: Deoxysugars, Aminosugars Carbohydrates are the most abundant compounds found in nature (cellulose: 100 billion tons annually) General characteristics Most carbohydrates are found naturally in bound form rather than as simple sugars (Glycoconjugates) Polysaccharides (starch, cellulose, inulin, gums), Mucopolysaccharides (hyaluronic acid) Glycoproteins and proteoglycans (hormones, blood group substances, antibodies) Glycolipids (cerebrosides, gangliosides) Nucleic acids (Ribose and desoxyribose) Functions Sources of energy (Glucose, Fructose,..) Intermediates in the biosynthesis of other basic biochemical entities (Fats and proteins) Form structural tissues in plants and in microorganisms (cellulose, lignin, murein) Participate in cell-cell recognition, Cell-cell adhesion Classification of carbohydrates Monosaccharides (monoses or glycoses) Oligosaccharides (condensation products of two to ten monosaccharides) Di, tri, tetra, penta, up to 9 or 10 Most important are the disaccharides Polysaccharides or glycans (more than 10) Homopolysaccharides Heteropolysaccharides Classification of Monosaccharides A. Number of carbons in chain Trioses (3C) tetroses (4C) pentoses (5C) hexoses (6C) heptoses (7C) B. Aldose or Ketose Aldoses (e.g., glucose) have an aldehyde group at one end Ketoses (e.g., fructose) have a keto group, usually at C2 Aldose sugars H (H C O C OH)n CH2OH Aldose H H H H C O C OH CH2OH Aldotriose n=1 C O H C OH H C OH CH2OH Aldotetrose n=2 H C O H C OH H C H C C O H C OH OH H C OH OH H C OH H C OH CH2OH Aldopentose n=3 CH2OH Aldohexose n=4 Ketose sugars CH2OH (H C O C OH)n CH2OH C O CH2OH CH2OH Ketose H C O C OH CH2OH Ketotriose n=0 CH2OH CH2OH Ketotetrose n=1 C O CH2OH H C OH C O H C OH C OH H CH2OH H Ketopentose n=2 H OH C OH CH2OH Ketohexose n=3 Sugars Exhibit Various Forms of Isomerism Aldose and Ketose isomers L and D Isomers D Form: Most of the monosaccharides occurring in mammals are D sugars, and the enzymes responsible for their metabolism are specific for this configuration. L form: 1. L-arabinose 2. L-idoronic acid Enantiomers Epimers Two sugars that differ only in the configuration around one carbon atom are called epimers; Optical isomerism Asymmetric compounds rotate plane polarized light In general, a molecule with n chiral centers can have 2n stereoisomers Of the 16 possible aldohexoses, eight are D forms and eight are L Most of the hexoses of living organisms are D isomers. POLARIMETRY Measurement of optical activity in chiral or asymmetric molecules using plane polarized light Measurement uses an instrument called a polarimeter (Lippich type) Rotation is either (+) dextrorotatory or (-) levorotatory polarimetry Magnitude of rotation depends upon: 1. The nature of the compound 2. The length of the tube (cell or sample container) usually expressed in decimeters (dm) 3. The wavelength of the light source employed; usually either sodium line at 589.3 nm or mercury vapor lamp at 546.1 nm 4. Temperature of sample 5. Concentration of analyte in grams per 100 ml Polarimetry [] T D observed x 100 = lxc D = Na+ line T = temperature oC obs : observed rotation in degree (specify solvent) l = length of tube in decimeter c = concentration in grams/100ml [] = specific rotation Specific rotation of various carbohydrates at 20oC D-glucose +52.7 D-fructose -92.4 D-galactose +80.2 L-arabinose +104.5 D-mannose +14.2 D-arabinose -105.0 D-xylose +18.8 Lactose +55.4 Sucrose +66.5 Maltose+ +130.4 Invert sugar -19.8 Dextrin +195 Racemic mixture In chemistry, a racemic mixture, or racemate, is one that has equal amounts of left- and righthanded enantiomers of a chiral molecule. A racemic mixture is denoted by the prefix (±)- or dl(for sugars the prefix DL- may be used), indicating an equal (1:1) mixture of dextro and levo isomers A racemate is optically inactive Tartaric acid • • • It occurs naturally in many plants, particularly grapes, bananas Add to foods to give a sour taste Used as an antioxidant levotartaric dextrotartaric acid acid (D-(−)(L-(+)tartaric acid) tartaric acid) mesotartaric acid Structural representation of sugars Fisher projection: straight chain representation Haworth projection: simple ring in perspective Conformational representation: chair and boat configurations Rules for drawing Haworth projections draw either a six or 5-membered ring including oxygen as one atom O Pyran O Furan most aldohexoses are six-membered Aldotetroses, Aldopentoses, ketohexoses are fivemembered Rules for drawing Haworth projections Next number the ring clockwise starting next to the oxygen 5 O 1 4 3 O 2 1 4 3 2 If the substituent is to the right in the Fisher projection, it will be drawn down in the Haworth projection (Down-Right Rule) Rules for drawing Haworth projections For D-sugars the highest numbered carbon (furthest from the carbonyl) is drawn up. For L-sugars, it is drawn down For D-sugars, the OH group at the anomeric position is drawn down for α and up for β. For L-sugars α is up and β is down Formation of the two cyclic forms of D-glucose Anomers: Isomeric forms of monosaccharides that differ only in the configuration about the hemiacetal or hemiketal carbon atom are called Mutarotation: interconvert of The and b anomes of Dglucose in aqueous solution <1% Conformations: interconvertible without the breakage of covalent bonds, configurations : interconvertible only by breaking a covalent bond—for example, in the case of α and β configurations, one-third two-thirds Condensation reactions: acetal and ketal formation D-ribose and other five-carbon saccharides can form either furanose or pyranose structures Chair and boat conformations of a pyranose sugar 2 possible chair conformations of b-D-glucose Envelope Conformations of β - D-ribose Envelope Conformations Oxidation reactions Aldoses may be oxidized to 3 types of acids ◦ Aldonic acids: aldehyde group is converted to a carboxyl group ( glucose – gluconic acid) ◦ Uronic acids: aldehyde is left intact and primary alcohol at the other end is oxidized to COOH Glucose --- glucuronic acid Galactose --- galacturonic acid ◦ Saccharic acids: (glycaric acids) – oxidation at both ends of monosaccharide) Glucose ---- saccharic acid Galactose --- mucic acid Mannose --- mannaric acid Solutions of cupric ion (known as Fehling's solution) provide a simple test for sugars such as glucose. Sugars that react are called reducing sugars; those that do not are called nonreducing sugars Reduction either done catalytically (hydrogen and a catalyst) or enzymatically the resultant product is a polyol or sugar alcohol (alditol) glucose form sorbitol (glucitol) mannose forms mannitol fructose forms a mixture of sorbitol glyceraldehyde gives glycerol Sructures of some sugar alcohols used as a sugar substitute in foods, especially for diabetics. In cosmetics it is commonly used in aftershave lotions, mild soaps and baby shampoos. Sorbitol is used as a humectant and skin conditioning agent Special monosaccharides: deoxy sugars These are monosaccharides which lack one or more hydroxyl groups on the molecule one quite ubiquitous deoxy sugar is 2’-deoxy ribose which is the sugar found in DNA 6-deoxy-L-mannose (L-rhamnose) is used as a fermentative reagent in bacteriology examples of deoxysugars Several sugar esters important in metabolism Special monosaccharides: amino sugars Constituents of mucopolysaccharides The anomeric forms of methyl-D-glucoside Properties Differences in structures of sugars are responsible for variations in properties Physical Crystalline form; solubility; rotatory power Chemical Reactions (oxidations, reductions, condensations) Physiological Nutritive value (human, bacterial); sweetness; absorption Oligosaccharides Most common are the disaccharides Sucrose, lactose, maltose, terehalose Maltose : 2 molecules of D-glucose Lactose : glucose + galactose Sucrose : glucose + fructose Terehalose : 2 molecules of D-glucose Sucrose α-D-glucopyranoside + β-D-fructofuranoside commercially obtained from sugar cane or sugar beet hydrolysis yield glucose and fructose (invert sugar) (sucrose: +66.5o ; glucose +52.5o; fructose –92o) used pharmaceutically to make syrups Sources of sucrose Sugar cane Sugar beet Structure of sucrose Non reducing sugar Lactose β-D-galactose (β 1,4) α-D-glucose used in infant formulations, medium for penicillin production and as a diluent in pharmaceuticals Reducing sugar Maltose 2-glucose molecules joined via α(1,4) linkage known as malt sugar produced by the partial hydrolysis of starch (either salivary amylase or pancreatic amylase) used as a nutrient (malt extract; Hordeum vulgare); as a sweetener and as a fermentative reagent Structure of maltose Glc(α1 4)Glc reducing sugar Trehalose Non reducing sugar Lactulose Galactose-β-(1,4)-fructose A semi-synthetic disaccharide (not naturally occurring) Not absorbed in the GI tract Used either as a laxative Metabolized in distal ileum and colon by bacteria to lactic acid, formic acid and acetic acid (remove ammonia) Oligosaccharides Trisaccharide: raffinose (glucose, galactose and fructose) Tetrasaccharide: stachyose (2 galactoses, glucose and fructose) Pentasaccharide: verbascose (3 galactoses, glucose and fructose) raffinose Raffinose can be hydrolyzed to D-galactose and sucrose by the enzyme α-galactosidase (α-GAL), an enzyme not found in the human digestive tract. α-GAL also hydrolyzes other α-galactosides such as stachyose, verbascose, and galactinol, if present. The enzyme does not cleave β-linked galactose, as in lactose. stachyose (2 galactoses, glucose and fructose) verbascose (3 galactoses, glucose and fructose) Polysaccharides or glycans homoglycans (starch, cellulose, glycogen, inulin) heteroglycans (mucopolysaccharides) characteristics: polymers (MW from 200,000) White and amorphous products (glassy) not sweet not reducing; do not give the typical aldose or ketose reactions) form colloidal solutions or suspensions Homoglycans Starch most common storage polysaccharide in plants composed of 10 – 30% a-amylose and 70-90% amylopectin depending on the source the chains are of varying length, having molecular weights from several thousands to half a million Amylose and amylopectin are the 2 forms of starch. Amylopectin is a highly branched structure, with branches occurring every 12 to 30 residues Amylose and amylopectin, the polysaccharides of starch amylopectin Strands of amylopectin form double helical structures with each other or with amylose strands Salivary amylase : α (1-4) endoglycosidase G 1-4 link G G G G G G G G G G G G G G amylase 1-6 link G G G G G G Limit G G G G G G G G dextrins maltotriose G G G maltose G G isomaltose Dextrins produced by the partial hydrolysis of starch along with maltose and glucose used as mucilages (glues) also used in infant formulas (prevent the curdling of milk in baby’s stomach) Cellulose Polymer of β-D-glucose attached β) linkages Yields glucose upon complete hydrolysis Partial hydrolysis yields cellobiose Most abundant of all carbohydrates Cotton flax: 97-99% cellulose Wood: ~ 50% cellulose The structure of cellulose (linear, unbranched homopolysaccharide) Structure of cellulose Glycogen also known as animal starch stored in muscle and liver present in cells as granules (high MW) contains both a(1,4) links and a(1,6) branches at every 8 to 12 glucose unit complete hydrolysis yields glucose hydrolyzed by both a and b-amylases and by glycogen phosphorylase Inulin β-(1,2) linked fructofuranoses linear only; no branching Jerusalem artichokes lower molecular weight than starch hydrolysis yields fructose sources include onions, garlic, dandelions and jerusalem artichokes used as diagnostic agent for the evaluation of glomerular filtration rate (renal function test) Chitin Chitin is the second most abundant carbohydrate polymer Present in the cell wall of fungi and in the exoskeletons of crustaceans, insects and spiders Chitin is used commercially in coatings (extends the shelf life of fruits and meats) Heteropolysacharides Glycosaminoglycans (Mucopolysacharides) they are the polysaccharide chains of proteoglycans they are linked to the protein core via a serine or threonine (O-linked) the chains are linear (unbranched) the glycosaminoglycan chains are long (over 100 monosaccharides) they are composed of repeating disaccharides Glycosaminoglycans Involved in a variety of extracellular functions; chondroitin is found in tendons, cartilage and other connective tissues Glycosaminoglycans A characteristic of glycosaminoglycans is the presence of acidic functionalities (carboxylate and/or sulfates) Glycosaminoglycans Proteoglycans are glycosaminoglycan-containing macromolecules of the cell surface and extracellular matrix Proteoglycan structure Some proteoglycans can form proteoglycan aggregates Aggrecan interacts strongly with collagen in the extracellular matrix of cartilage, contributing to the development and tensile strength of this connective tissue Proteoglycan aggregate of the extracellular matrix Proteoglycans are a family of glycoproteins whose carbohydrate moieties are predominantly glycosaminoglycans structures are quite diverse as are sizes examples: versican, serglycin, decorin, syndecan Functions: - modulate cell growth processes - provide flexibility and resiliency to cartilage Hyaluronate: material used to cement the cells into a tissue Interactions between cells and the extracellular matrix Cross-linked meshwork that gives the whole extracellular matrix strength and resilience Bacterial cell wall provide strength and rigidity for the organism consists of a polypeptide-polysaccharide known as petidoglycan or murein determines the Gram staining characteristic of the bacteria Structure of peptidoglycan Structure of peptidoglycan Cell wall of Gram-positive bacteria Gram-negative bacteria Cross-section of the cell wall of a gram-negative organism such as E.coli Lipopolysaccharide Lipopolysaccharide (LPS) coats the outer membrane of Gram-negative bacteria. the lipid portion of the LPS is embedded in the outer membrane and is linked to a complex polysaccharide Glycoproteins (Mucoprotins) Usually done as a post-translational process Proteins can contain either O-linked oligosaccharides or N-linked oligosaccharides Serine or threonine O-linked saccharides Aspargine N-linked glycoproteins Roles of oligosaccharides in recognition and adhesion at the cell surface Complex structure (branching, stereoisomerism) •Hexasaccharid: 1.05*1012, hexapeptide: 4096, hexanucleotide: 6.4*107 Clone, Expresion, PCR-amplification Sequencing synthesis Lectins Lectins are glycoproteins that recognize and bind to specific oligosaccharides. Recognition/binding of CHO moieties of glycoproteins, glycolipids & proteoglycans by animal lectins is a factor in: cell-cell recognition adhesion of cells to the extracellular matrix recognition of disease-causing microorganisms initiation and control of inflammation. Glycoarray Glycome: • to describe the complete rang of oligosaccharide sequences that can be found in a cell type, organ or organism Glycomics: • study of glycome by glycoarray کدام یک از قند های زیر دارای کربن نامتقارن کمتری است؟ الف) گلوکز ب) فروکتوز ج) ریبوز د) ریبولوز کدام قند قادر به احیا مس نیست؟ الف) ترهالوز ب) گلوکز ج) مالتوز د) فروکتوز کدام پلی ساکارید از تکرار – Nاستیل گلوکز آمین حاصل می شود؟ د) نشاسته ج) کیتین ب) هپارین الف) اینولین