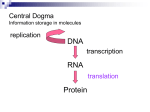
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Ppt
Personalized medicine wikipedia , lookup
Molecular ecology wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Genetic engineering wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Epitranscriptome wikipedia , lookup
Biochemistry wikipedia , lookup
Biosynthesis wikipedia , lookup
Institute of Molecular Biotechnology Swetlana Nikolajewa and Thomas Wilhelm Institute of Molecular Biotechnology Jena, Germany, http://www.imb-jena.de/tsb Jena We present a new classification scheme of the genetic code, based on a binary representation of purines (A, G = 1) and pyrimidines (U, C = 0). On the basis of this logical organization we detect codon regularities (strong, mixed and weak codons) and patterns of amino acid properties and show symmetry characteristics of the genetic code. We present the “codon - reverse codon” pattern and use it to remove the only ambiguity which had remained in our recently proposed new classification scheme of the genetic code. The purine-pyrimidine scheme has now found its final form. We show that the “codon - reverse codon” pattern is correlated to aspects of the tRNA anticodon distribution in all organisms. It is well known that there is no tRNA with an anticodon for any of the STOP codons; we found that there is also no tRNA containing reverse STOP anticodons. The four combinations of the first column (CC*, GC*, CG*, and GG*) always imply 6 hydrogen bonds in complementary base-paring with the corresponding anticodon of the tRNA. Accordingly, they are called strong codons. The classification scheme of the genetic code is based on a binary representation of purines (G,A) - 1, pyrimidines (C,U) - 0. The first two bases guarantee 5 H-bonds: mixed codons. The eight rows (23 = 8) and four columns are sufficient to place the 20 amino acids, as well as the termination codons. Code The common table of the standard genetic code Second Letter First Letter U C UUU A UCU Phe G UAU Ser Third Letter UGU Tyr UCC UAC UGC UUA UCA UAA UGA Stop A UGG Trp G UUG UCG CUU CCU Leu C C CGU His CCC CAC CGC C CUA CCA CAA CGA A Leu Pro CCG AUU ACU Ile AUC AUA ACA AUG Met GUU ACG GUC GCC 4 hydrogen bonds Pro CC Ser UC (C/U) Leu (C/U) CU (C/U) Phe UU (C/U) Pro CC (A/G) Ser UC (A/G) Leu CU (A/G) Leu UU 100 Ala GC (C/U) Thr AC (C/U) Val GU (C/U) Ile AU 101 Ala GC (A/G) Thr AC (A/G) Val GU (A/G) Ile / Met AU 010 Arg CG (C/U) Cys UG (C/U) His CA (C/U) Tyr UA 011 Arg CG (A/G) Gln CA (A/G) Stop UA (A/G) 110 Gly GG (C/U) Asp GA (C/U) Asn AA (A/G) (C/U) (A/G) (C/U) A G GGU Asp GAC 5 hydrogen bonds Arg AGG GAU Ala C AGA Lys AAG GCU Val U Ser AGC AAA Thr 5 hydrogen bonds U Gly GGC C Stop / Trp UG (A/G) G GUA GCA Val GUG GAA Ala GCG GGA Glu GAG Interestingly, in 5 different mitochondrial codes (including yeast) AU(A/G) always encodes for Met and UG(A/G) encodes Trp. Thus, 32 positions code for 32 entries and each row contains exactly four different amino acids. In this spirit the mitochondrial code is the most regular one. G AGU Asn AAC 6 hydrogen bonds Each row contains exactly 4 different amino acids (including the stop codon). Exceptions in the standard code are the second row with two leucines and the fourth row with the AU* start codon. Arg CGG AAU Thr ACC Ile Gln CAG Weak codons U Arg CUC CUG A CAU Pro Mixed codons 001 Stop UAG Mixed codons U UUC Ser Strong codons Cys U Leu 000 Weak codons have just 4 H-bonds . A The codon-anticodon symmetry: the red horizontal line marks the symmetry axis. For instance, codon CCC (Pro, first column, first row) has the anticodon GGG (Gly, first column, last row). Gly GGG G 111 Gly GG Ser AG (A/G) Arg (C/U) AG In 8 different mitochondrial codes UG(A/G) encodes Trp. (A/G) Glu GA (A/G) Lys AA (C/U) (A/G) The arrows represent “codon - reverse codons” pairs. The point symmetry corresponds to Halitsky’s family – nonfamily symmetry operation (“E-M bifurcation”, Halitsky 2003). For instance, the family codon GUA (Val) is mapped into the nonfamily codon UGC (Cys). Thus, this point symmetry is behind the family – nonfamily symmetry in our scheme (shaded vs. unshaded regions). “Codon - Reverse Codon” Pattern The arrows in the above table indicate “codon - reverse codons” pairs: the reverse codon of a codon XYZ is defined as ZYX, where X, Y, Z can be any base. For instance, the reverse codon of GGA (Gly) is AGG (Arg). There exist 15 different amino acids in the rows 000, 101, 010 and 111 where the codon is reverse to itself, e.g. Lys (AAA), Tyr (UAU). Stop 1. The table can be divided into four blocks (codon - reverse codon groups) of the same size, for instance the upper left block with Pro (P), Ser (S), Ala (A) and Thr (T). Each block shows the same arrow pattern. All strongly evolutionary conserved groups of amino acids (Thompson et al., 1994) are subsets of exactly one codon - reverse codon group, e.g. the MILV amino acids belong to the upper right block in the table. The other conserved strong groups belonging to one block are STA, NEQK, NHQK, NDEQ, QHRK, MILF, HY. The only exception is FYW. 2. We studied all known tRNA genes of 104 different organisms (Sprinzl et al., 1999). It is known that the STOP codons do not have any tRNA. We found that there are also no tRNA genes containing anticodons reverse to the STOP anticodons (ACT, ATC and ATT in Table 2). The only exception is H. sapiens, possessing one tRNAAsn gene with the anticodon ATT, but humans also have three different possible suppressor tRNA genes (Lowe and Eddy, 1997). AUC UAG STOP Asp CUA GAU Asp Table 2. The amino acid codon- reverse codon pairs and the number of tRNA genes for the corresponding anticodons. Codons AA Reverse Codons Anticodons # tRNAs AA Anticodons # tRNAs Stop UGA TCA 0 Ser AGU ACT 0 Stop UAG CTA 0 Asp GAU ATC 0 Stop UAA TTA 0 Asn AAU ATT 1 Tyr UAC GTA 189 His CAU ATG 0 Trp UGG CCA 144 Gly GGU ACC 0 Leu UUA TAA 127 Ile AUU AAT 1 Leu UUG CAA 134 Val GUU AAC 18 Phe UUC GAA 154 Leu CUU AAG 28 Ser UCA TGA 151 Thr ACU AGT 19 Ser UCC GGA 116 Pro CCU AGG 16 3. There are some anticodons, where the reverse anticodon has no tRNA, e.g. all 104 studied organisms have at least one tRNA for Tyr with anticodon GTA (some organisms, for instance H.sapiens, have different tRNAs with the same anticodon, altogether there are 189 different tRNAs with the anticodon GTA in the 104 species), but no organism has a tRNA for His with anticodon ATG. Table 2 lists different codon - reverse codon pairs. In the whole superkingdom bacteria the number of tRNA genes for the reverse part of table 2 is always zero, the few exceptions are only in eukaryotes and archaea. Another unique property of all bacteria is that there is no tRNA with anticodons for the self reverse codons UUU (Phe), UCU (Ser), UGU (Cys) and UAU (Tyr). Generally, table 2 shows that anticodons with an A at the third position are strongly preferred, whereas A** anticodons are significantly suppressed. REFERENCES Crick, F. H. C. (1968) The origin of the genetic code. J. Mol. Biol., 38, 367- 379. Halitsky, D. (2003) Extending the (hexa-)rhombic dodecahedral model of the genetic code: the code’s 6-fold degeneracies and the orthogonal projections of the 5-cube as 3-cube. Contributed paper (983-92-151), American Mathematical Society; and personal communication. Jungck, J.R. (1978) The genetic code as a periodic table. J. Mol. Evol., 11, 211-224. Lagerkvist, U. (1978) “Two out of three”: An alternative method for codon reading. Proc. Natl Acad. Sci. USA, 75, 1759-1762. Lagerkvist, U. (1981) Unorthodox codon reading and the evolution of the genetic code. Cell, 23, 305-306. Lowe, T. M., Eddy, S. R. (1997) tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res., 25, 955-964. http://lowelab.ucsc.edu/GtRNAdb/ Nikolajewa, S., Beyer, A., Friedel, M., Hollunder, J., and Wilhelm, T. (2005) Common patterns in type II restriction enzyme binding sites. Nucleic Acids Res., 33, 2726-2733. Sprinzl, M., Vassilenko, K. S., Emmerich, J., Bauer, F., (1999) Compilation of tRNA sequences and sequences of tRNA genes. Last update 2003. www.uni-bayreuth.de/departments/biochemie/trna/ . Roberts, R.J., Vincze, T., Posfai, J., Macelis, D. (2005) REBASE—restriction enzymes and DNA methyltransferases Nucleic Acids Res., 33, D230–D232 Thompson, J. D., Higgins, D. G., Gibson T. J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res., 22, 4673-4680. Wilhelm, T., Nikolajewa, S. L. (2004) A new classification scheme of the genetic code. J. Mol. Evol., 59, 598 -605 . Woese, C. R. (1967) The genetic code: The molecular basis for Genetic Expression. Harper & Row, New York.