* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download metabolism - Farmasi Unand
Survey
Document related concepts
Transcript
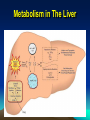
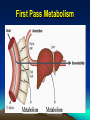
METABOLISM Dr. Muslim Suardi, MSi., Apt. Faculty of Pharmacy University of Andalas 2008 ELIMINATION “The irreversible removal of drug from the body by all routes of elimination” • Metabolism • Excretion METABOLISM Biotransformation The process by which the drug is chemically converted in the body to a metabolite Enzymatic Non Enzymatic (ester hydrolysis) Place of Metabolism • • • • • • • Mainly: Liver Kidneys Lung Small Intestine Skin GI mucosal cells Microbiological flora in the distal portion of the ileum & large intestine Metabolism in The Liver Clearance “The process of drug elimination from the body or from the single organ without identifying the individual processes involved” The volume of fluid cleared of drug from the body per unit of time (mL/min) BIOTRANSFORMATION REACTIONS Active drug to inactive metabolite Amphetamine Phenylacetone Active drug to active metabolite Codeine Morphine Inactive drug to active metabolite Hetacillin Ampicllin Active drug to reactive intermediate PCT Reactive metabolite REACTIONS • Phase I More polar metabolites A-synthetic reactions • Phase II Conjugation Synthetic reactions Much more polar metabolites Phase I • Occurs first • Introduce or expose a functional group on drug mol • Oxygen into phenyl group of phenylbutazone by aromatic hydroxylation to form oxyphenbutazone • Codeine is demethylated to form morphine • Hydrolysis of ester Aspirin to form Salicylic Acid Phase I • Oxidation Aromatic hydroxylation Side chain hydroxylation N-, O-, and S-dealkylation Deamination Sulfooxidation, N-oxidation N-hydroxylation • Reduction Azoreduction Nitroreduction Alcohol dehydrogenase • Hydrolysis Ester hydrolysis Amide hydrolysis Phase II • Salicylic Acid with glycine to form salicyluric acid • Salicylic Acid with glucoronic acid to form salicylglucoronide • Conjugating reagents • Derived from biochemical compounds involved carbohydrate, fat, & protein metabolism Phase II • • • • • • Glucuronidation Sulfation Amino acid conj Acetylation Methylation Glutathione conj by by by by by by Glucuronic acid Sulfate Glycine Acetyl CoA CH3 glutathione Hepatic Elimination K = km + ke The rate constant of elimination (K) = first-order rate constant for metabolism (km) + the first-order rate constant for excretion (ke) Clinical Focus • The overall el half life (t1/2) of a drug is 2 hr (K=0.3465/hr, km=0.104/hr). • If the renal excretion pathway becomes impaired as in the case of certain kidney disorders, then less or none drug will be excreted renally & hepatic metabolism may become the primary drug elimination route. Clinical Focus K but ke thus k = km + ke, = 0, = km. T1/2 = 0.693/k. t1/2 = 0.693/0.104 = 6.7 hr Variation of Biotransformation Enzymes in Humans • • • • • Genetics Factors Environmental Factors Drug Interactions Physiologic Conditions Drug Dosage Regimen Genetics Factors • Genetic different within population • Racial differences among different populations Environmental Factors Enzyme induction Enzyme inhibition Drug Interactions Enzyme induction Enzyme inhibition Physiologic Conditions Age Gender Diet/Nutrition Pathophysiology Drug Dosage Regimen Route of drug administration Dose dependence (nonlinear) pharmacokinetics. Pharmacogenetics • • • • • “Genetic differences in drug elimination “ INH N-acetylation Rapid & slow acetylation Slow acetylation neurotoxicity The differences is referred to as genetic polymorphism. Genetic Polymorphism • Procainamide acetylated • Hydralazin acetylated • Glucose-6-phosphate-dehydrogenase deficiency, approximately 10% of black Americans • Phenytoin, Efficient & Poor Metabolizer • Propranolol, difference among Chinese population DI Involving Drug Metabolism The enzymes involved in the metabolism of drugs may be altered by: • Diet • Co-administration of other drugs & chemicals Enzyme Induction A drug or chemical-stimulated increase in enzyme activity usually due to an increase in the amount of enzyme present • Eg.: Phenobarbital Carbamazepine Rifampicin Benzopyren (Smoking) Chlordane (Insecticide) Enzyme Inhibition • May be due to substrate competition or due to direct inhibition of drug metabolizing enzyme, particularly one of several of the cytochrome P-450 enzymes. • Eg.: Fluoxetine decrease the Cl of IMI due to its inhibitory effect of hydroxylation. Inhibition Inhibitors Example PCT EtOH Cimetidine Erythromycin Result Increased hepatotoxicity Warfarin Prolongation of prothrombin time Carbamazepine Decreased Carbamazepine Cl Induction Inducers of drug metabolism Carbamazepine Example Result PCT Increased PCT metabolism Rifampin Methadone Increased methadone metabolism First Pass Metabolism First Pass Effect • Routes of administration may affects the metabolic rate • A drug given parenterally, transdermally, or by inhalation may distribute within the body prior to metabolism by the liver. First Pass Effect • In contrast, drugs given orally are normally absorbed in the duodenal segment of the small intestine and transported via the mesenteric vessels to the hepatic portal vein & then to the liver prior to the systemic circulation. First Pass Effect • Drugs that are highly metabolized by the liver or by the intestinal mucosal cells demonstrate poor systemic availability when given orally. • This rapid metabolism of an orally administered drug prior to reaching the general circulation is termed FPE or presystemic elimination.