* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 38_Chromoproteins. Pathological and physiological forms of h
Photosynthetic reaction centre wikipedia , lookup
Biosynthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Point mutation wikipedia , lookup
Siderophore wikipedia , lookup
Human iron metabolism wikipedia , lookup
Biochemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
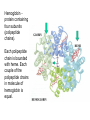
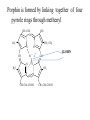
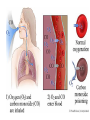
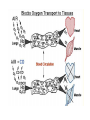
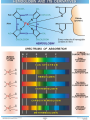
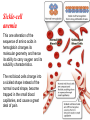
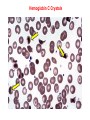
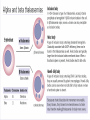
Chromoproteins • In our organism are present: complex proteins - include Fe2+ - cytochrome oxidase(heme), hemoglobin , mioglobin, catalase, peroxidase, Cu2+ - cytochrome oxidase, Zn2+ carbonic anhidrase, Mg2+ hexokinase, piruvate kinase and etc. Respiratory pigments • Hemoglobin Erythrocytes derive their colour from a complex protein called hemoglobin. This substance is composed of a pigment, heme, containing iron, and the protein glohin. Hemoglobin has the power to attract oxygen molecules and to hold them in a loose chemical combination known as oxyhemoglobin. It is said, therefore, to have a chemical affinity for oxygen. Quantity and chemical structure of hemoglobin • In man – 130-160 g/L; • in woman – 120-140 g/L. • It consists of four folded polypeptide chains of amino acid units. The four chains form the globin, or protein, part of the molecule. In addition there are four atoms of iron, each associated with a pigment, or heme, group of atoms. The heme group provides the red colour of the blood and also its oxygencombining ability. The iron atoms are bivalent or in the ferrous state. There are 2 pairs of polypeptides in each hemoglobin molecule, 2 of the subunits containing one type of polypeptide and 2 containing another. Hemoglobin protein containing four subunits (polipeptide chains). Each polipeptide chain is bounded with heme. Each couple of the polipeptide chains in molecule of hemoglobin is equal. • The main type of hemoglobin in blood of adult people (96 %) is the form containing two alpha and two beta chains. It is the adult hemoglobin or HbA1 or α2β2. Except this form there is about 2 % of hemoglobin A2 (α2δ2) and 2-3 % of fetal hemoglobin (α2γ2) Porphin is formed by linking together of four pyrrole rings through methenyl bridges. CH2=CH2 CH CH3 H3C CH2=CH2 N N ++ CH Fe N GLOBIN CH N H3C CH3 CH CH2-CH2-COOH CH2-CH2-COOH Biosynthesis of heme The major main in the biosynthesis of heme are the liver and the erythrocyte - producing cells of the bone marrow, which are active in hemoglobin synthesis. I. Formation of A - aminolevulinic acid . All the carbon two simple bluding blocks; glycine and succinyl CoA. Glycine and succinyl CoA condense to form A.L.A in a reaction catalyzed by ALA synthase. This reaction requires pyridoxal phosphate as a coenzyme vitamin B6, and is the rate-controlling step in porphyrin biosynthesis. By deficiency By deficiency of the iron Fe is develops - iron deficiency anemia or hypochromic anemia. Prophyrias - are caused by inherited or acquired defects in heme synthesis, resulting in the accumulation and increased excretion or porphyrins or porphyrin precurcors. The porphyries are classified as erythropoietic or hepatic depending on whuther the enzyme deficiency occurs in red blood cells or the liver. Metabolism of iron • Daily requirements of for our organism in the iron Fe=10-20 mg. • From total iron - 65 - 70% in the structure hemoglobin 20% - contain myoglobin contain 1% - in the structure cytrochromes, cytrochromoxidase heme contain enzymes • 10-15% - in the liver , bone marrow. • Transport of iron ensure specific protein transferrin transport form of iron . • In the structure this protein the iron has valency - Fe 3+ and joins with anion hydrocarbonate. Ferritin • Ferritin - helps to store iron in certain tissues / liver, spleen, bone marrow • Ferririn consists of 24 subunits arranged in the form of a shell around iron atoms Fe2+. One apoferritin molecule encloses more than 2000-3000 ferric atoms. With passage of time lysosomal enzymes degrade ferritin to hemosiderin which is a molecule of non-specific structure / a mixture of partially degraded protein, lipid, iron. Hemosiderin Hemosiderin another reserve form of iron. By excess of iron level of hemosiderin in the liver increase and develops hemosiderosis of liver damage the liver. Idiopatic hemochromatosis is often inherited disease. In primary hemochromatosis there is excessive accumulation of iron in tissues. Thus results in tissue damage. In the liver iron accumulation can cause cirrosis. In the pancreas in can damage beta - cells resulting in diabetes mellitus. Iron accumulation in skin can cause pigmentation of skin bronze colour. Thus the condition is called bronze diabetes. Respiratory pigments Myoglobin • Hem is also part of the structure of myoglobin, an oxygen-binding pigment found in red (slow) muscles and in the respiratory enzyme cytochrome c. Porphyrins other than that found in hem play a role in the pathogenesis of a number of metabolic diseases (congenital and acquired porphyria, etc.) It may be the reserve pigments, which give the tissue oxygen in a small oxygen condition. Hemoglobin derivates 1. Physiological hemoglobin derivatives • Oxy Hb - it is used for of transport of O2 in the body (HbO2). • Hb NH COOH- carbhemoglobin 2. Pathological derivatives of hemoglobin • /carboxyhemoglobin/ -Hb - CO • Met-Hb Fe 3+ Physiological HbCO2 HbNHCOOH is used for transfer of CO2 from peripheral tissues to the lungs (carbhemoglobin, HbCO2) Methemoglobin • The iron ion may either be in the Fe2+ or Fe3+ state, but ferrihemoglobin (methemoglobin) (Fe3+) cannot bind oxygen. In binding, oxygen temporarily oxidizes (Fe2+) to (Fe3+), so iron must exist in the +2 oxidation state to bind oxygen. The enzyme methemoglobin reductase reactivates hemoglobin found in the inactive (Fe3+) state by reducing the iron center. Carboxyhemoglobin • The binding of oxygen is affected by molecules such as carbon monoxide (CO) (for example from tobacco smoking, car exhaust and incomplete combustion in furnaces). CO competes with oxygen at the heme binding site. • Hemoglobin binding affinity for CO is 200 times greater than its affinity for oxygen, meaning that small amounts of CO dramatically reduce hemoglobin's ability to transport oxygen. When hemoglobin combines with CO, it forms a very bright red compound called carboxyhemoglobin, which may cause the skin of CO poisoning victims to appear pink in death, instead of white or blue. Abnormal hemoglobin Abnormal hemoglobins are the result of mutations in the genes that code for α or β chains of globin. Hemoglobinopathias Sickle-cell anemia (HbS) and hemoglobin C disease (HbC) are the classical examples of abnormal hemoglobin. The structure of hemoglobin contains two α- and two β-globin chains. • Molecular basis of HbS In case of sickle-cell anemia, the hemoglobin (HbS) has two normal α-globin and two abnormal β-globin chains. This is due to a difference in a single amino acid. In HbS, glutamate at sixth position of β-chain is replaced by valine Thalassemias, on the other hand, are caused by decreased synthesis of normal hemoglobin Sickle-cell anemia This one alteration of the sequence of amino acids in hemoglobin changes its molecular geometry and hence its ability to carry oxygen and its solubility characteristics. The red blood cells change into a sickled shape instead of the normal round shape, become trapped in the small blood capillaries, and cause a great deal of pain. Hemoglobin C Crystals