* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Survey
Document related concepts
Transcript
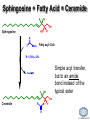
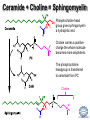
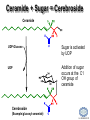
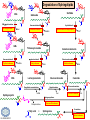
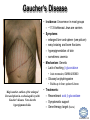
LIPID MAPS Lipid Metabolomics Tutorial Sphingolipids Professor Edward A. Dennis Department of Chemistry and Biochemistry Department of Pharmacology, School of Medicine University of California, San Diego Copyright/attribution notice: You are free to copy, distribute, adapt and transmit this tutorial or individual slides (without alteration) for academic, non-profit and non-commercial purposes. Attribution: Edward A. Dennis (2010) “LIPID MAPS Lipid Metabolomics Tutorial” www.lipidmaps.org E.A. DENNIS 2010 © Sphingolipid definitions Sphingosine: a family of compounds, with the most common found in mammals being this 18-carbon amino alcohol with a trans double bond; the starting point for ceramides. Ceramide: a sphingosine molecule connected to a fatty acid by an amide bond. Ceramides are the starting point for sphingomyelin, cerebrosides and gangliosides. Sphingomyelin: a ceramide that has a phosphorylcholine head group in place of its hydroxyl. Present in most mammalian cells, and rich in myelin sheaths around nerves. E.A. DENNIS 2010 © Palmitoyl CoA + Serine = Sphingosine Serine Palmitoyl-CoA 1. Serine donates 2 carbons and an amino group 2. Reduction of the carbonyl to a hydroxyl 3. Acyl group added to convert to a dihydroceramide 4. Then, oxidation to add a double bond Look familiar? Resembles both b-oxidation and D4 desaturation N-acyl-Sphingosine (Ceramide) E.A. DENNIS 2010 © Sphingosine-1-phosphate +H N 3 Sphingosine kinase Sphingosine phosphatase • Sphingosine can be phosphorylated by sphingosine kinases, ubiquitous enzymes in the cytosol, ER and nucleus to make sphingosine-1-phosphate (S1P). • Sphingosine-1-phosphate, a lysophospholipid, acts as a potent messenger molecule that operates both intra- and inter-cellularly. • Within the cell, it promotes mitosis and inhibits apoptosis. It also regulates calcium mobilization and cell growth in response to a variety of extracellular stimuli. +H N 3 • • Outside the cell, S1P exerts many of its effects through interaction with five specific G protein-coupled receptors on cell surfaces. Different cells have different receptor profiles. S1P is vital to the function of several immune cells. It is a major regulator of T cell development, B and T cell recirculation, tissue homing patterns, and chemotactic responses to chemokines. E.A. DENNIS 2010 © Sphingosine + Fatty Acid = Ceramide Sphingosine Fatty acyl-CoA R = (CH2)n-CH3 Simple acyl transfer, but to an amide bond instead of the typical ester Ceramide E.A. DENNIS 2010 © Ceramide + Choline = Sphingomyelin Phosphocholine head group gives sphingomyelin a hydrophilic end Choline carries a positive charge the whole molecule becomes more amphoteric The phosphocholine headgroup is transferred to ceramide from PC Choline E.A. DENNIS 2010 © Comparison of Sphingomyelin and PC At least one fatty acid of PC is usually unsaturated or polyunsaturated, whereas, SM is usually saturated or mono-unsaturated; therefore, SM rich membranes are less “fluid” than typical PC-rich membranes. E.A. DENNIS 2010 © Comparison of S-1-P and LPA Sphingosine-1-phosphate (neutral zwitterion; net charge 0) +H N 3 Lysophosphatidic acid (Example: 1-myristoyl-sn-glycerophosphate) (net negative charge) E.A. DENNIS 2010 © More Definitions Galactose (polar head) Ceramide (non-polar tail) Glycosidic bond Cerebrosides: a ceramide that has a sugar added to the head group. Most commonly, the sugar is glucose (Glu) or galactose (Gal). Sialic acid Gangliosides: a ceramide that has multiple sugars including at least 1 sialic acid residue added to the head group. Increased variety and complexity. E.A. DENNIS 2010 © Ceramide + Sugar = Cerebroside Ceramide UDP-Glucose Sugar is activated by UDP UDP Addition of sugar occurs at the C1 OH group of ceramide Cerebroside (Example:glucosyl-ceramide) E.A. DENNIS 2010 © Ceramide + (Many Sugars) = Gangliosides GM1 GM2 GM3 Sugars are activated by UDP (sialic acid by CMP) Each sugar is added individually Gangliosides can have varied, complex structures They often function as antigens and surface markers Stearic acid (C18) N-acyl chain Trivia: Do you know your blood type? Is it A+? B-? O? The letters refer to the specific multi-sugar structures are attached to gangliosides and proteins on the surface of your red blood cells. E.A. DENNIS 2010 © Degradation of Sphingolipids • The amide bond of sphingolipids does not break down easily – which is why they make good membrane components • Enzymatic degradation is used for turnover – LOTS of degradation enzymes exist • it’s a long, complicated bunch of pathways • Genetic defects in these enzymes cause a long list of diseases – – – – all involve unhealthy accumulation of some sphingolipid most are rare, but more common in specific ethnicities key diseases: Gaucher’s, Tay-Sachs’, Fabry’s and Niemann-Pick Resources: (Online Mendelian Inheritance in Man) • OMIM Web site: www.ncbi.nih.gov/OMIM/searchomim.html E.A. DENNIS 2010 © Degradation of Sphingolipids GM1 Sulfatide Globoside GM1 b -galactosidase Hexosaminidase A/B GM1 Gangliosidosis Gal GM2 GM3 Metachromatic leukodystrophy GalNAc Trihexosylceramide GalNAc Fabry’s disease Lactosylceramide Ganglioside neuraminidase Galactocerebrosidase Krabbe’s disease Gal Glucocerebroside b -galactosidase NANA Sphingomyelin SO42- Galactocerebroside a -galactosidase A Hexosaminidase A Tay-Sachs disease Sandhoff’s disease Arylsulfatase A Gal Gal Ceramide Glucocerebrosidase Gaucher’s disease Glc Sphingomyelinase Phosphocholine Fatty acid + Niemann-Pick disease Sphingosine Ceramidase Farber’s disease E.A. DENNIS 2010 © Tay-Sachs’ Disease • Incidence: Like Gaucher’s but rarer – ~1:30 Ashkenazi Jews are carriers – ~1:500 carriers in general population • Symptoms:Neurodegenerative – mental retardation and seizures – listlessness, fixed gaze, hypotonia – cherry-red spot on retina (see picture) • Mechanism: Genetic – Lack of GM2 hexosaminidase A Cherry-red spot on a patient’s retina, a common finding in patients with TaySachs’ disease. • Auto recessive, OMIM #272800 – Ganglioside GM2 • Builds up in CNS • Treatments: No good therapy yet Trivia: Injections of recombinant hexosaminidase A do not help Tay-Sachs’ patients because it cannot cross the blood-brain barrier. – Supportive and symptomatic – Patients die by age 5 – Gene therapy target (future) E.A. DENNIS 2010 © Gaucher’s Disease • Incidence: Uncommon in most groups – ~1:13 Ashkenazi Jews are carriers • Symptoms: – enlarged liver and spleen (see picture) – easy bruising and bone fractures – hyperpigmentation of skin – sometimes: anemia • Mechanism: Genetic – Lack of working b-glucosidase • Auto recessive, OMIM #230800 – Glucosyl acylsphingosine • Builds up in liver, spleen & bone Magic marker outlines of the enlarged liver and spleen in a school-aged boy with Gaucher’s disease. Note also the hyperpigmented skin. • Treatments: – Recombinant acid b-glucosidase – Symptomatic support – Gene therapy target (future) E.A. DENNIS 2010 © Niemann-Pick Disease Type A • Incidence: Type A is the most severe of the 5 subtypes of Niemann-Pick Disease – ~1:90 Ashkenazi Jews are carriers • Symptoms: Neurodegenerative – Large abdomen within 3-6 mos. and jaundice – Progressive loss of early motor skills, progressive spasticity, developmental delay – Cherry red spot in the eye – (Generally) a very rapid decline leading to death by two to three years of age. • Mechanism: Genetic – Lack of Sphingomyelinase • Auto recessive, OMIM #257200 – Sphingomyelin Patient with Niemann Pick Disease • builds up in CNS, liver and lungs • Treatments: – Supportive and symptomatic – Patients die by age 3 – No effective therapy to date E.A. DENNIS 2010 © Summary of Today’s Sphingolipids Molecule(s) Synthesis Scheme Significance Sphingosine Palmitoyl CoA + Serine Brings in the amine group Important signaling molecule Ceramides Sphingosine + Fatty Acid Amide bond, hydrophobicity Important signaling molecule Sphingomyelins Ceramide + PhosphoCholine Amphoteric & charged, diseases Membrane component Cerebrosides Ceramide + (Mono)saccharides Amphoteric & neutral, diseases Rich in brain Gangliosides Ceramide + Polysaccharides + Sialic acid Complexity, diseases Rich in brain E.A. DENNIS 2010 © Acknowledgement This tutorial is based on an evolving subset of lectures and accompanying slides presented to medical students in the Cell Biology and Biochemistry course at the School of Medicine of the University of California, San Diego. I wish to thank Dr. Bridget Quinn and Dr. Keith Cross for aid in developing many of the original slides, Dr. Eoin Fahy for advice in applying the LIPID MAPS nomenclature and structural drawing conventions [Fahy et al (2005) J Lipid Res, 46, 839-61; Fahy et al (2009) J Lipid Res, 50, S9-14] and Masada Disenhouse for help in adopting to the tutorial format. Edward A. Dennis September, 2010 La Jolla, California E.A. DENNIS 2010 ©