* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 2: Chemistry of Life 2.1: Atoms, Ions, and Molecules
Survey
Document related concepts
Transcript
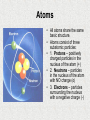
Chapter 2: Chemistry of Life 2.1: Atoms, Ions, and Molecules • Warm Up: What complex things do you know that are made up by simple units? • Objectives: Identify elements common to living things. • Describe how ions form. • Compare ionic and covalent bonding. • Terms to Know: Atom, elements, compound, ion, ionic bond, covalent bond, molecule. Living things Consist of Atoms of Different Elements • An Atom is the smallest basic unit of matter. • Millions of atoms can fill the space of a pencil dot on a sheet of paper. Atoms • All atoms share the same basic structure. • Atoms consist of three subatomic particles: • 1. Protons – positively charged particles in the nucleus of the atom (+) • 2. Neutrons – particles in the nucleus of the atom with NO charge (o) • 3. Electrons – particles surrounding the nucleus with a negative charge (-) Elements • An Element is one particular type of atom, and it cannot be broken down into simpler substances by ordinary chemical means. • Elements are arranged on the Periodic Table and each has it’s own unique characteristics. – Ex: hydrogen, oxygen, carbon, nitrogen, phosphorus Atoms and Elements • All atoms of a given element have a specific number of protons, neutrons and electrons. • The number of protons and electrons is given by the Atomic number of the element. – Ex: Hydrogen’s atomic # is 1, so it has 1 proton and 1 electron. – Ex: Oxygen’s atomic # is 8, so it has 8 protons and 8 electrons. • The number of neutrons can be found by subtracting atomic number from atomic mass. – Ex: Hydrogen’s atomic mass is 1 so the number of neutrons is 1-1 or 0 – Ex: Oxygen’s atomic mass is 16 so the number of neutrons is 16-8 or 8 • Valence Electrons are those found in the outermost shell of an atom. – The magic number for valence electrons is 8. Compounds • A Compound is a substance made of atoms of different elements bonded together in a certain ratio. • Common compounds in living things include water (H2O) and carbon dioxide (CO2). • Compounds commonly have different properties than the elements that make them up. – Ex: hydrogen and oxygen are gases but combined they for water, a liquid. • How are elements different from compounds? Ions form when Atoms Gain or Lose Electrons • An Ion is an atom that has gained or lost one or more electrons. • An Ion forms because an atom is more stable when its outermost energy level is full; the gain or loss of electrons allows this to occur. • Some ions are positively charged (those that lose electrons) • Some ions are negatively charged (those that gain electrons). • An Ionic Bond form through the electrical force between oppositely charged ions. – Ex: Salt is an ionic bond between sodium and chlorine. – Chlorine (Cl) has 7 valence electrons (needs 1) and Sodium (Na) has 1 valence electron (need to get rid of it) – Sodium donates its electron to chlorine forming an ionic bond that creates salt. Ionic Bonds Atoms Share Pairs of Electrons in Covalent Bonds • Not all atoms gain or lose electrons. • Many elements must share electrons to fill their valence shells. • A Covalent Bond is formed when two or more atoms share pairs of electrons. – Ex: Carbon has 4 valence electrons (needs 4) and Oxygen has 6 valence electrons (needs 2). – Neither atom can donate electrons so they share pairs to fill the valence shell. • A Molecule is two or more atoms held together by covalent bonds. – Ex: Water, Carbon Dioxide, Glucose. • What happens to electrons in outer energy levels when two atoms form a covalent bond? Covalent Bonds 2.2: Properties of Water • Objectives: Recognize the importance of hydrogen bonding. • Explain why many compounds dissolve in water. • Compare acids and bases. • Warm Up: Why is water so important to life? • Words to Know: Hydrogen bond, cohesion, adhesion, solution, solvent, solute, acid, base, pH. Water and Hydrogen Bonds • • • • • • Water is a Polar Molecule, this means that is has a slightly positive end and a slight negative end (like a magnet). Polar molecules form when atoms in a molecule have unequal pulls on the electrons they share. Other molecules, called nonpolar molecules, do not have these charged regions. Opposite charges of polar molecules interact to form Hydrogen Bonds. A Hydrogen Bond is an attraction between a slightly positive hydrogen atom and a slightly negative atom. Hydrogen Bonding is very common in water, but also occurs in other molecules. Properties Related to Hydrogen Bonding • • Water is a liquid at the temperature that support most life on Earth. Hydrogen bonds give water a high Specific Heat, meaning water resists changes in temperatures. – This process is crucial to cells where homeostasis is required. • Cohesion is the attraction among molecules of the same substance (water to water). – Ex: Water forming bead on a window. • Adhesion is the attraction of molecules of different substances. – Ex: Water sticks to glass in a graduated cylinder. • How are hydrogen bonds similar to ionic bonds? Many Compounds Dissolve in Water • • • • • • A Solution is a mixture of substances that is the same throughout – it is a homogenous mixture. A Solution is made up of two parts: – The Solvent is the substance that is present in the greater amount and dissolves the other substance. – The Solute is the substance that dissolves in the solvent. – Ex: Koolaid: Solvent: water, Solute: Koolaid – Ex: Blood Plasma: Solvent: water, Solute: sugars and proteins. Nonpolar substances such as fats and oils, rarely dissolve in water. Nonpolar substances will dissolve in other nonpolar substances. Polar substances will dissolve in water. What are the solvent and solutes in a beverage you drink? Some Compounds form Acids or Bases • • • • • Some compounds break up into ions when they dissolve in water. An Acid is a compound that releases a proton – Hydrogen Ion (H+) – when it dissolves in water. – An acid increases the amount of H+ ions in solution. A Base is a compound that remove H+ ions from solution. – In these cases, more OHions in the solution. A solution’s acidity, or H+ concentration is measure on a pH Scale. pH is usually measured between 0 – 14. – 0 is very acidic, 14 is very basic and 7 is neutral. pH Scale Buffers • Most organisms need to keep their pH within a very narrow range around neutral (7). • One way pH is regulate in organisms is by substances called Buffers. • A Buffer is a compound that can bind to an H+ ion when the H+ ion concentration increases and can release H+ ions when the H+ concentration decreases. • Cells have higher H+ concentrations than blood. Which has the higher pH? Why? 2.3 Carbon-Based Molecules • Objectives: Describe the bonding properties of carbon atoms. • Compare carbohydrates, lipids, proteins, and nucleic acids. • Warm Up: What do you think of when you hear the word organic? • Words to Know: Monomer, Polymer, Carbohydrate, Lipid, Fatty Acid, Protein, Amino Acid, Nucleic Acid Carbon Atoms have Unique Bonding Properties • • • • • • • • Carbon atoms are the basis of most molecules that make up living things. Carbon can form covalent bonds with up to 4 other atoms, including other carbon atoms. Carbon-Based molecules have three fundamental structures: 1. Straight Chain 2. Branched Chain 3. Ring Monomer – Small single units that link together to make larger molecules. Polymer – a large molecule, or macromolecule, made of many monomers bonded together. Straight Chain Branched Chain Ring Four Main Types of CarbonBased Molecules are Found in Living Things Monomer Polymer Examples Monosaccharides (simple sugars) Polysaccharides (Carbohydrates) Glucose, Fructose, Starch Triglyceride (3 fatty acids and a glycerol) Lipids Fats, oils, and waxes. Amino Acids Protein Muscle, hair, nails Nucleotides Nucleic Acids DNA, and RNA Carbohydrates • Carbohydrates are molecules composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio. • These include sugars and starches. • Carbohydrates serve as the Main energy source for living things. • The most basic carbohydrates are simple sugars or Monosaccharides. – Ex: Glucose, Fructose • Many glucose molecules can be linked to form Polysaccharides. – Ex: Starches, Glycogen, Cellulose. • • • • • Lipids Lipids are nonpolar molecules that include fats, oils, wax, and cholesterol. Lipids make up the cell membrane (phospholipids). Lipids provide protection and insulation for the cell. Lipids can also be used as an energy source. The main types of Lipids are Saturated and Unsaturated Fats. – – Saturated Fats – contains all single bonds and is a solid at room temperature. Unsaturated Fats – contain at least one double bond and are liquids at room temperature. Proteins • • • • • • • • The most varied of the carbon-based molecules. Proteins are used for structure. A Protein is a polymer made of monomers called amino acids. Amino Acids are molecules that contain Carbon, Hydrogen, Oxygen, Nitrogen, and sometimes Sulfur. Organisms use 20 different amino acids to build proteins. Amino acids are composed of: A hydrogen atom, an amino group (NH2), and a carboxyl group (COOH). Amino acids only differ in their side group of R-group. Amino acids are held together by peptide bonds. – • Ex: muscle, hair, nails, enzymes. Enzymes are protein catalysts that help speed up reactions. Nucleic Acids • Nucleic Acids are polymers that are made up of monomers called nucleotides. • A nucleotide is composed of a sugar, a phosphate, and a nitrogen base. • Ex: DNA and RNA • Nucleic Acids create the detailed instructions for creating proteins. 2.4 Chemical Reactions • Objectives: Describe how bonds break and reform during chemical reactions. • Explain why chemical reactions release or absorb energy. • Words to Know: Chemical Reaction, Reactant, Product, Bond Energy, Equilibrium, Activation Energy, Exothermic, Endothermic, Atom, Molecule Reactants, Products and Bond Energy • Reactants are the substances that go into a chemical reaction. • Products are the substances created by the reaction. • 6O2 + C6H12O6 6CO2 + 6H2O Reactants Products Chemical Reactions Release or Absorb Energy • Activation Energy is the amount of energy that needs to be absorbed for a chemical reaction to start. • An Exothermic Reaction releases energy. • An Endothermic Reaction absorbs energy. Chemical Reactions Release or Absorb Energy 2.5 Enzymes • Objectives: Explain the effect of a catalyst on activation energy. • Describe how enzymes regulate chemical reactions. • Words to Know: Catalyst, Enzyme, Substrate A Catalyst Lowers Activation Energy • A catalyst is a substance that decreases the activation energy needed to start a reaction. • Catalysts are not considered to be a reactant or product in the reaction. Enzymes • Enzymes are catalysts for chemical reaction in living things. • Enzymes lower the activation energy needed to start chemical reactions. • Enzymes are involved in almost every process in living things. • Conditions such as temperature and pH can effect how well enzymes work. • Enzyme structure is important because each enzyme’s shape allows only certain reactants to bind to the enzyme. Enzymes cont… • The specific reactants that an enzyme acts on are called Substrates. • This model is called lock and key sine all components must fit together just right for the enzyme to work. Enzymes cont… • Scientists have recently discovered that the structure of enzymes are not fixed in one place. • Enzymes can actually bend slightly when they are bound to their substrates. • This is known as Induced fit.