* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Scaglione
Survey
Document related concepts
Transcript
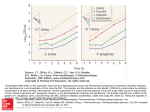
PK/PD: TOWARDS DEFINITIVE CRITERIA PK/PD in clinical Practice: new level of PK/PD Francesco Scaglione Department of Pharmacology, Toxicology and Chemotherapy, University of Milan, Milan, Italy PK/PD results and evolution Persistend effect } Time/Conc. dependent activity 2000 ? Improvement Outcome of dose and resistance intervals } Several objectives of PK/PD resistance Phase 2-3 clinical trial Improvement of therapy PK/PD evolution 2000 Custom-made therapy In Vitro and in vivo activity A D M E Effect over the time; Peculiar Effects concentration (µg/ml) Pharmacology : what for physician? 20 18 16 14 12 10 8 6 4 2 0 0 1 2 3 4 5 6 7 time h 8 9 10 11 12 Considerations when choosing an antibacterial agent Microbiology Mechanism of action Concentration at infection site Antibacterial spectrum Outcome Drug Pathogen MIC PK Absorption Distribution Metabolism Excretion Optimal dosing regimen Clinical efficacy Bacterial eradication Compliance with dosing regimen Time- vs concentrationdependent killing Tolerability Rate of resolution Bactericidal vs bacteriostatic activity Prevention of resistance Tissue penetration Persistence of antibacterial effect PD PK/PD parameters affecting efficacy Concentration Peak/MIC AUC/MIC Time >MIC MIC PAE 0 Time (hours) Improving the probability of positive outcomes IMPROVING THE ODDS HOST BUG DRUG PK/PD parameters determining efficacy Absorption Serum levels Distribution and penetration to site of infection Intracellular penetration Relationship of PK parameters to MIC In clinical practice Infections are treated with the same dosing regimen irrespective of the absolute susceptibility of the microrganisms as well as the PK of the actual patient Aminoglycoside dosing characteristics for 78 patients with pneumonia caused by gram-negative bacteria Variable Aminoglycoside dose (mg)a Cmax (µg/ml)a Cmin (µg/ml)a a Before IPM (n = 78) After IPM (n = 60) 105 (90-140) 230 (175-320) 5.3 (3.9-6.3) 6.7 (5.2-7.6) 0.6 (0.3-1.1) 0.8 (0.5-1.1) Values are medians (interquartile ranges). Adapted from Angela D. M. Kashuba; AAC 1999 Aminoglycoside pharmacokinetic and pharmacodynamic variables for 78 patients with pneumonia caused by gram-negative bacteria Variable Median (interquartile range) Aminoglycoside CL (ml/min/1.73 m2) 71.5 (50.4-91.3) Aminoglycoside half-life (h) AUC0-24 (µg · h/ml) 3.5 (2.6-5.0) 52.2 (34.5-77.5) First Cmax/MIC 3.6 (1.4-6.2) Second Cmax/MIC 3.7 (1.9-6.9) Adapted from Angela D. M. Kashuba; AAC 1999 Peak level of tobramycin 3mg/kg in ten patients in ICU 10.0 mg/L 7.5 5.0 2.5 0.0 FACTORS INVOLVED IN INFLAMMATION TNF- a IL - 1 IL - 6 Trauma Necrosis Bacteria PMN MN Lymphocytes PAF PGE LTC TXA endothelial Damage Increase of capillary permeability Protease Complement oxygen Radicals Oedema VARIATIONS OF INTERSTITIAL FLUID DURING INFECTIONS blood INTERSTITIAL FLUID Cells THEORETICAL CONCENTRATION OF AN ANTIBIOTIC Concentration Serum Interstitial fluid Large volume compartment Time Vd of Tobramicin in 13 patients admitted in ICU 50 40 L 30 20 10 0 0 1 2 3 4 days 5 6 7 mg/L Serum peak of Tobramicin in 13 patients admitted in ICU 10.0 7.5 5.0 2.5 0.0 0 1 2 3 4 days 5 6 7 Concentration Time Over MIC Peak/MIC M IC2 M IC1 ‘Time above MIC’ Time Ideal approach to adjust the dose Initial dosing regimen(chosen by patient’s physician) Blood sampling( two or more postdistributional sample) Pharmacokinetic analysis (peak,AUC,CL) Adjust dose or/and intervals (PK/PD) Redetermine concentrations Adjust again ? First problem PK approach to adjust the dose is poor applicable for routinely use (at moment) N°samples Personnel Costs Microbiology second problem PK/PD breakpoints betalactams (ceftriaxone) aminoglicosides quinolones glicopeptides macrolides tetraciclines Program to customize the therapy in our hospital Isolation of the pathogen and MIC Design therapy traditionally(by patient’s physician) Pharmacokinetic Adjust dose or interval using PK/PD Redetermine concentrations PK/PD values adopted •Aminoglicosides Peak/MIC 8 •Quinolones peak/MIC 10 •Betalactams peak/MIC 4 and T>MIC 70% same value for monotherapy or combination Sampling time •Aminoglicosides Peak : 0.5 h from end 30 min infusion •Quinolones peak : 0.5 h from end 60 min infusion •Betalactams peak :0.5 h from end 30 min infusion And T>MIC : 5.6 hours from start infusion mg/L Concentrations of ceftazidime and cefotaxime in serum 65 60 55 50 45 40 35 30 25 20 15 10 5 0 C 0.5 h C 5.6 h Peak levels of amikacin mg/L 40 30 20 10 0 PK/PD dose adjustment Levofloxacin 500 mg to 750 OD or BID Ciprofloxacin 500mg to 750 BID Cefotaxime-Ceftazidime 2g q 8 to 2g q6 Amikacin 15 mg/kg OD to 20 mg/kg OD* * Patients are daily monitored for safety preliminary results October 2000 – April 2001 Patients included 680 Evaluated for PK/PD 223 (32.8%) Dose or interval adjusted 84 (37.7%) Adjustment failed in 6 (5 cipro -1 amikacin) diagnosis Nosocomial pneumonia 105 Sepsis 44 upper UTI 57 Necrotizing Fascitis 8 Others 9 Organisms isolated Pseudomonas aeruginosa 87 Stenotrophomonas spp 4 Staphylococcus aureus 42 Legionella species 2 Enterobacter species Citrobacter species 2 33 Klebsiella species 15 Acinetobacter 1 Escherichia coli 14 Proteus species 1 Haemophilus influenzae 11 Serratia marcescens 7 Streptococcus pneumoniae 4 outcome Length failure hospitalization - days* PK/PD 11 (7-16) 39/223 analysed (17.5%) PK/PD Not analysed 16 (9-23 ) *From the diagnosis of infection 147/457 (31.9 %) hospitalization days correlation between time to adjust the dose and hospitalisation days 20 15 10 5 0 0 25 50 75 100 125 150 hours to adjust doses 175 Conclusions I The PK/PD approach may: improve the outcome shorten the time to clinical improvement Reduce the length of hospitalisation Conclusions II The initial higher costs for analysis and personnel are compensate for the reduction of the hospitalisation, with a financial gain Conclusions III Can PK/PD be used in everyday clinical practice? yes