* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 1 - Drugs and Agents - Factors Affecting their Action
Psychedelic therapy wikipedia , lookup
Specialty drugs in the United States wikipedia , lookup
Pharmaceutical marketing wikipedia , lookup
Compounding wikipedia , lookup
Orphan drug wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Drug design wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Drug discovery wikipedia , lookup
Neuropharmacology wikipedia , lookup
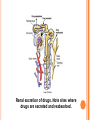
Study of the history, sources, and physical and chemical properties of drugs Also looks at the ways in which drugs affect living systems Various subdivisions of pharmacology have evolved Study of the biochemical and physiological effects of drugs Study of drugs’ mechanisms of action Study of the absorption, distribution, biotransformation (metabolism), and excretion of drugs Four steps Absorption Distribution Metabolism Excretion Study of how drugs may best be used in the treatment of illnesses Study of which drug would be most appropriate or least appropriate to use for a specific disease; what dose would be required; etc. The study of drugs derived from herbal and other natural (plant and animal) drug sources Studying compositions of natural substances helps to gain knowledge for developing synthetic versions Study of poisons and poisonings All drugs have the potential to become toxic. Ancient Egypt: the cradle of pharmacology These medical sources listed over 700 different remedies for different ailments. First century: Dioscorides prepared De Materia Medica: Listed and classified 600 different plants used for medicinal purposes; first time plants were ever classified Drugs derived from: Natural sources Semisynthetic sources Synthetic sources Symptomatic treatment Prevention Diagnostic drugs Curative Health maintenance Contraception Tablets Timed or sustained release Tablets or controlled release Capsule Troches Suppositories Solutions Douche Suspensions Emulsions Topicals Patches Drug implants Ampules Sterile Sealed glass or plastic container Contain a single liquid dose Vials: either single or multiple dose Glass or plastic container Sterile liquid dose Sealed with a rubber diaphragm Chemical name The drug’s chemical composition and molecular structure Generic name (nonproprietary name) Name given by the United States Adopted Names Council Allows the drug to be marketed Chemical name Generic name (+/-)-2-(p-isobutylphenyl) propionic acid Ibuprofen Trade name Motrin Also called trade name (proprietary name) The drug has a registered trademark; use of the name is restricted by the drug’s owner (usually the manufacturer) Allows the drug to be commercially distributed The superscript ® is registered by the U.S. Patent Office and approved by the FDA (Food and Drug Administration) Pure Food and Drug Act of 1906 Required all drugs to meet minimal standards Federal Food, Drug, and Cosmetic Act of 1938 Required the drug to be safe before being distributed over state lines (continues) 1970: Comprehensive Drug Abuse Prevention and Control Act Also known as Controlled Substance Act: classified drugs according to their abuse potential Regulates the manufacture and distribution of drugs causing dependence CONTROLLED SUBSTANCES SCHEDULES Schedule I High potential for abuse No medical use Heroin LSD Schedule II High potential for abuse Accepted medical use Morphine Demerol Schedule III Lower potential for Accepted medical abuse use Librium, Valium, hydrocodone, Tylenol with codeine Schedule IV Lower potential for Accepted medical abuse use Librium Valium Schedule V Lowest potential for abuse Lomotil Robitussin A-C Accepted medical use Prescription drugs = legend drugs Drugs prescribed by: Physician Nurse practitioner Physician’s assistant Dentist Veterinarian Others Drugs Alter existing cellular or chemical functions Exert their action by forming a chemical bond with specific receptors within the body Referred to as a lock and key effect Drug receptor interaction. Binding with specific receptors occurs only when the drug and its receptors have a compatible chemical shape. Receptors The better the fit, the stronger the drug’s affinity, thus Drug effect occurs at lower doses Agonist effect Antagonistic effect Adverse drug effect Therapeutic effect Routes Oral Parenteral Topical The metabolism of a drug and its passage from the liver into the circulation Metabolism occurs in the liver Liver enzymes react with the drug Increases the dosage requirement The same drug—given IV—bypasses the liver, preventing the first-pass effect from taking place, and more drug reaches the circulation. The transport of a drug in the body by the bloodstream to its site of action The elimination of drugs from the body Kidneys (main organ) Liver Bowel Renal excretion of drugs. Note sites where drugs are secreted and reabsorbed. Half-life The time it takes for one half of the original amount of a drug to be removed from the body