* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Enzyme Mechanisms
Survey
Document related concepts
Transcript
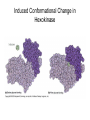
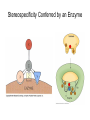
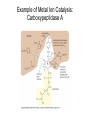
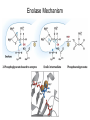
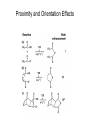
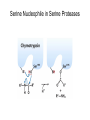
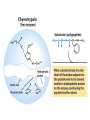
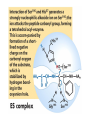
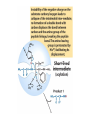
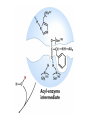
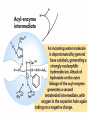
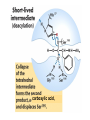
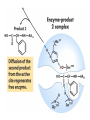
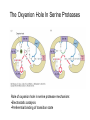
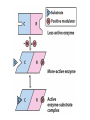
Enzyme Mechanisms 1. 2. 3. 4. 5. 6. Oxidoreductases catalyze oxidation-reduction reactions. Transferases catalyze transfer of functional groups from one molecule to another. Hydrolases catalyze hydrolytic cleavage. Lyases catalyze removal of a group from or addition of a group to a double bond, or other cleavages involving electron rearrangement. Isomerases catalyze intramolecular rearrangement. Ligases catalyze reactions in which two molecules are joined. Types of Enzymes Two Models for Enzyme-Substrate Interaction Induced Conformational Change in Hexokinase Coenzymes Example of a Coenzyme Involved in Oxidation/Reduction Reactions Hydride (H:-) transfer (nicotinamide adenine dinucleotide) Stereospecificity of Yeast Alcohol Dehydrogenase ethanol acetaldehyde pro-S pro-R pro-R pro-S Vennesland and Westheimer, 1950 Stereospecificity Conferred by an Enzyme Catalytic Mechanisms 1. 2. 3. 4. 5. 6. Acid-base catalysis Covalent catalysis Metal ion catalysis Electrostatic catalysis Proximity and orientation effects Preferential binding to transition state (transition state stabilization) Acid-Base Catalysis Keto-Enol Tautomerism: Uncatalyzed vs. Acid- or Base-Catalyzed Example of Acid-Base Catalysis: Bovine Pancreatic RNase A Covalent Catalysis: Nucleophiles and Electrophiles Protonated Example of Covalent Catalysis: Decarboxylation of Acetoacetate Lysine side chain e-amino group on enzyme is nucleophile in attack on substrate. Electrophilic “electron sink” Example of Metal Ion Catalysis: Carboxypeptidase A Example of Metal Ion Catalysis: Carbonic Anhydrase Carbonic anhydrase catalyzes the reaction: CO2 + H2O HCO3− + H+ Enolase Mechanism Entropic and Enthalpic Factors in Catalysis Proximity and orientation effects Transition state stabilization through preferential binding of transition state Proximity and Orientation Effects Enzymes Are Complementary to Transition State Serine Protease Mechanism: Multiple Catalytic Mechanisms at Work Structure of the Serine Protease Chymotrypsin Serine Protease Substrate Specificity and Active-Site Pockets Trypsin cleaves amide bond immediately Cterminal to basic amino acid residues. Substrate specificity in serine proteases through active-site binding of side chain of amino acid residue adjacent to amide bond that will be cleaved. Chymotrypsin cleaves amide bond immediately C-terminal to hydrophobic amino acid residues. Serine Nucleophile in Serine Proteases The Pre-Steady State in ChymotrypsinCatalyzed Hydrolysis of p-Nitrophenyl Acetate P1 k1 H 2O E+S ES EP2 E + P2 k k k -1 vo = kcat[E]t[S]/(KM + [S]) Steady-state velocity, where kcat = k2k3/(k2 + k3) KM = KSk3/(k2 + k3) KS = k-1/k1 For chymotrypsin with ester substrates: k2 >> k3 Release of P1 faster than EP2 breaks down to E + P2 2 3 Serine Protease Mechanism Catalytic triad: •Acid-base catalysis •Covalent catalysis •Proximity/orientation effects •Also (not depicted here) - electrostatic catalysis and transition state stabilization carboxylic acid, The Oxyanion Hole In Serine Proteases Role of oxyanion hole in serine protease mechanism: •Electrostatic catalysis •Preferential binding of transition state Trypsin/Bovine Pancreatic Trypsin Inhibitor (BPTI) Complex Trypsin-BPTI complex resembles tetrahedral transition state. Transition State in Proline Racemase Reaction and Transition State Analogs Proline racemase preferentially binds transition state, stabilizing it, and is potently inhibited by transition state analogs. RNA-Based Catalysts (Ribozymes) Cleavage of a Typical Pre-tRNA by Ribonuclease P Ribonuclease P is a ribonucleoprotein (RNA- and protein-containing complex), and the catalytic component is RNA. An even more complex example of an RNA- and protein-containing enzyme system is the ribosome. The central catalytic activity of the ribosome (peptide bond formation) is catalyzed by an RNA component. tRNA substrate of ribonuclease P Catalysis by the Intervening Sequence in Tetrahymena Preribosomal RNA RNA by itself without any protein can be catalytic. Enzyme Regulation Effect of Cooperative Substrate Binding on Enzyme Kinetics Cooperative enzymes do not obey simple Michaelis-Menten kinetics. Effect of Extreme Homoallostery Homotropic allosteric regulation by substrate: S at one active site affects catalysis of S P at sites on enzyme other complex. Extreme positive cooperativity depicted here [S]c = critical substrate concentration Heteroallosteric Control of an Enzyme Heterotropic allosteric regulation: non-S effectors modulate catalysis of S P. (positive allosteric effector) (negative allosteric effector) A Model for Enzyme Regulation: Aspartate Transcarbamoylase (Aspartate Carbamoyltransferase) in Pyrimidine Synthesis Aspartate Transcarbamoylase (ATCase)Catalyzed Reaction Feedback Inhibition of ATCase by CTP Regulation of ATCase by ATP and CTP ATP is a positive heterotropic allosteric effector of ATCase, while CTP is a negative heterotropic allosteric effector. Detailed Structure of One Catalytic Subunit and Adjacent Regulatory Subunit of ATCase Quaternary Structure of ATCase in T State and R State CTP and ATP bind at regulatory site, but CTP preferentially binds in T state, while ATP preferentially binds R state. X-Ray Structure of Aspartate Transcarbamoylase T State T State “top” view “side” view R State “side” view