* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Type 2 Diabetes
Psychopharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Nicotinic agonist wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Neuropharmacology wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Discovery and development of dipeptidyl peptidase-4 inhibitors wikipedia , lookup
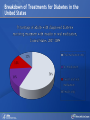
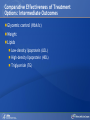
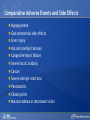
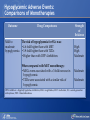
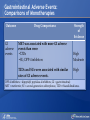
Comparing Medications for Adults With Type 2 Diabetes Prepared for: Agency for Healthcare Research and Quality (AHRQ) www.ahrq.gov Background: Type 2 Diabetes A chronic illness characterized by insulin resistance that is worsened by obesity and inactivity. Over time, pancreatic beta cells lose their ability to maintain the high insulin levels needed to counter liver and muscle insulin resistance and beta cell failure occurs. Background: Public Health Impact In the United States, a total of 25.8 million children and adults (8.3% of the population) have diabetes. Like many chronic illnesses, diabetes disproportionately affects older people and its prevalence is higher among racial and ethnic minority populations. The annual economic burden of diabetes is estimated to be $174 billion—$116 billion for direct medical costs and $58 billion for indirect costs (disability, work loss, premature mortality) After adjusting for population age and sex differences, average medical expenditures among people with diagnosed diabetes is 2.3 times higher than what expenditures would be in the absence of diabetes. CDC. National Diabetes Fact Sheet, 2011. www.cdc.gov. accessed 6/2011 Background: Complications Complications associated with long-standing diabetes include: Retinopathy and blindness Neuropathy Nephropathy End-stage kidney disease Increased risk of death from cardiovascular disease Background: Management Management of hyperglycemia is a very important part of treatment for type 2 diabetes to achieve improved macrovascular and microvascular outcomes. Controlling blood glucose levels for people with type 2 diabetes often requires several strategies. The clinical approach includes weight loss if needed, dietary control, increased physical activity, and antidiabetic medications (ADA-EASD). Source: 2007–2009 National Health Interview Survey. Background: Treatment Treatment regimens include monotherapy and combinations of two or three drugs from different classes. Eleven classes of diabetes medications are available: Biguanides Thiazolidinediones Sulfonylureas Dipeptidyl peptidase-4 (DPP-4) inhibitors Insulins Meglitinides Glucagon-like peptide-1 (GLP-1) receptor agonists An amylin analogue Alpha-glucosidase inhibitors Colesevalam (a bile-acid sequestrant) Bromocriptine Choosing among the available medications requires consideration of their benefits, mode of action/class, adverse effects, and cost. Breakdown of Treatments for Diabetes in the United States Rationale for Update In 2007, AHRQ published its first systematic review on the comparative effectiveness of oral medications for type 2 diabetes. In 2011, this review was updated to include newer medications and two-drug combination therapies: Noninsulin injectables: GLP-1 receptor agonists, exenatide, and liraglutide (FDA-approved in 2005 and 2010, respectively). DPP-4 inhibitors: sitagliptin and saxagliptin (FDA-approved in 2006 and 2009, respectively). Evidence about combinations of medications, including combinations of medications with insulin therapy. Agency for Healthcare Research and Quality (AHRQ) Comparative Effectiveness Review (CER) Development Topics are nominated through a public process, which includes submissions from health care professionals, professional organizations, the private sector, policymakers, the public, and others. A systematic review of all relevant clinical studies is conducted by independent researchers, funded by AHRQ, to synthesize the evidence in a report summarizing what is known and not known about the select clinical issue. The research questions and the results of the report are subject to expert input, peer review, and public comment. The results of these reviews are summarized into Clinician Research Summaries and Consumer Research Reviews for use in decisionmaking and in discussions with patients. The research reviews and the full report, with references for included and excluded studies, are available at: www.effectivehealthcare.ahrq.gov. Rating the Strength of Evidence From the CER The strength of evidence was classified into four broad categories: High Further research is very unlikely to change the confidence in the estimate of effect. Moderate Further research may change the confidence in the estimate of effect and may change the estimate. Low Further research is likely to change the confidence in the estimate of effect and is likely to change the estimate. Insufficient Evidence either is unavailable or does not permit estimation of an effect. Priority Medication Comparisons Monotherapy as main intervention Combination therapy as main intervention Main Intervention Comparisons Metformin • Thiazolidinedione, Sulfonylurea, DPP-4 inhibitor, Meglitinide, or a GLP-1 agonist • Combination of metformin plus thiazolidinedione • Combination of metformin plus sulfonylurea • Combination of metformin plus DPP-4 inhibitor • Combination of metformin plus meglitinide Thiazolidinedione • Different thiazolidinedione, Sulfonylurea, DPP-4 inhibitor, Meglitinide, or a GLP-1 agonist Sulfonylurea • DPP-4 inhibitor, Meglitinide, or a GLP-1 agonist DPP-4 inhibitor • DPP-4 inhibitor, Meglitinide, or a GLP-1 agonist Meglitinides • GLP-1 agonist Combination of metformin plus (a thiazolidinedione or a sulfonylurea or one of the meglitinides or a DPP-4 inhibitor or a GLP-1 agonist or a basal insulin or a premixed insulin) Combination of metformin plus (a thiazolidinedione or a sulfonylurea or a meglitinides or DPP-4 inhibitor or GLP-1 agonist or a basal insulin or a premixed insulin) Combination of metformin plus (a thiazolidinedione or a sulfonylurea or a meglitinides or DPP-4 inhibitor or GLP-1 agonist or a basal insulin or a premixed insulin) Combination of a thiazolidinedione plus (a sulfonylurea or a meglitinides or DPP-4 inhibitor or GLP-1 agonist) Results by Outcome Intermediate Outcomes Glycemic control or Hemoglobin A1c (HbA1c) Body weight LDL cholesterol HDL cholesterol Triglycerides Adverse events and side effects Mild to moderate hypoglycemia Gastrointestinal (GI) side effects Congestive heart failure Liver injury Hip and nonhip fractures Long-term clinical outcomes All-cause mortality Cardiovascular (CV) mortality Nonfatal myocardial infarction and stroke Microvascular outcomes (retinopathy, nephropathy, and neuropathy) Comparative Effectiveness of Treatment Options: Intermediate Outcomes Glycemic control (HbA1c) Weight Lipids Low-density lipoprotein (LDL) High-density lipoprotein (HDL) Triglyceride (TG) Intermediate Outcomes: Overview of HbA1c Results MET, SUs, TZDs, and Rep reduce HbA1c levels by about 1 percentage point (moderate to high strength of evidence). MET lowers HbA1c 0.4% better than do DPP-4 inhibitors (moderate strength of evidence). On average, two-drug combinations reduce HbA1c about 1 percentage point more than monotherapies (moderate to high strength of evidence). MET + SUs and MET + TZDs are equally effective at lowering HbA1c (moderate strength of evidence). Several other combinations therapies had similar efficacy at reducing HbA1c (low strength of evidence). DPP-4 inhibitors = dipeptidyl peptidase-4 inhibitors; HbA1c = hemoglobin A1c; MET = metformin; Rep = repaglinide; SU = second-generation sulfonylureas; TZD = thiazolidinediones. Intermediate Outcomes: Overview of Weight Results MET is associated with less weight gain when compared to other monotherapies or combinations (moderate to high strength of evidence). When compared to SUs, GLP-1 receptor agonists are associated with less weight gain (moderate strength of evidence). MET + SUs are associated with less weight gain than are combinations with TZDs (moderate strength of evidence). Some newer agents in combination show promise for lower levels of weight gain (low strength of evidence). DPP-4 inhibitors = dipeptidyl peptidase-4 inhibitors; GLP-1 receptor agonists = glucagon-like peptide-1 receptor agonists; MET = metformin; SU = second-generation sulfonylureas; TZD = thiazolidinediones. Intermediate Outcomes: Overview of Lipid Results In studies that lasted from 3 to 12 months, the effects of antidiabetic medications on lipid levels were generally small to moderate. MET had more favorable effects on HDL, LDL, and TG when compared to several monotherapy and combination therapy medications (moderate to high strength of evidence). Some treatments had mixed effects on lipid outcomes. HDL = high-density lipoprotein; LDL = low-density lipoprotein; MET = metformin; TG = triglycerides. Details of HbA1c and Weight Intermediate Outcomes: Comparisons of Monotherapies Outcomes HbA1c Weight Drug Comparisons MET, SU, TZD, and Rep reduce HbA1c by about 1%. Specific comparisons include: • MET versus SU • SU versus TZD; Pio versus RSG; MET versus TZD; SU versus Rep • MET lowers HbA1c 0.4% better than do DPP-4 inhibitors. Strength of Evidence High Moderate Moderate From the 2007 report, TZD, SU, and Rep increase weight by 1-5 kg, but MET did not increase weight in placebo-controlled trials. MET maintained or decreased weight when compared to other monotherapies as shown below: • MET versus TZD, -2.6 kg; MET versus SU, -2.7 kg • MET versus DPP-4 inhibitors, -1.4 kg GLP-1 receptor agonists were associated with less weight gain, by -2.5 kg, when compared to SUs. SU and MEG had similar effects on body weight. High Moderate Moderate High DPP-4 inhibitors = dipeptidyl peptidase-4 inhibitors; GLP-1 receptor agonists = glucagon-like peptide-1 receptor agonists; HbA1c = hemoglobin A1c; MEG = meglitinides; MET = metformin; Pio = pioglitazone; Rep = repaglinide; RSG = rosiglitazone; SU = second-generation sulfonylureas; TZD = thiazolidinediones. Details of the Lipid Intermediate Outcomes: Comparisons of Monotherapies Outcomes LDL HDL TG Drug Comparisons MET is associated with lower LDL levels than are: • SU, by -10.1 mg/dL • RSG, by -12.8 mg/dL; Pio, by -14.2 mg/dL; DPP-4 inhibitors, by -5.9 mg/dL Pio is associated with higher HDL levels when compared to: • MET, by 3.2 mg/dL • RSG, by 2.3 mg/dL; SU, by 4.3 mg/dL MET is associated with HDL levels similar to those of: • SU • RSG Strength of Evidence High Moderate High Moderate High Moderate Pio is associated with lower levels of TG by -27.2 mg/dL when compared to High MET. MET is associated with lower TG levels than were: • RSG, by -27 mg/dL Moderate • SU, by -8.6 mg/dL Moderate TG levels for SU and MEG were similar. Moderate DPP-4 inhibitors = dipeptidyl peptidase-4 inhibitors; HbA1c = hemoglobin A1c; HDL = high-density lipoprotein; LDL = lowdensity lipoprotein; MEG = meglitinides; MET = metformin; Pio = pioglitazone; RSG = rosiglitazone; SU = second-generation sulfonylureas; TG = triglycerides. Details of the HbA1c and Weight Intermediate Outcomes: Monotherapy versus Two-Drug Combination Therapy Outcome HbA1c Weight Drug Comparisons Strength of Evidence Two-Drug combination therapies are more effective than monotherapies, reducing HbA1c by an additional 1%. Specific comparisons include: • MET versus MET/SU • MET versus MET/TZD • MET versus MET/DPP-4 inhibitors High High Moderate MET has a more favorable effect on weight when compared to these combination therapies: • MET/TZD, by -2.2 kg • MET/SU, by -2.3 kg High High DPP-4 inhibitors = dipeptidyl peptidase-4 inhibitors; HbA1c = hemoglobin A1c; MET = metformin; SU = second-generation sulfonylureas; TZD = thiazolidinediones. Details of the Lipid Intermediate Outcomes: Monotherapy versus Two-Drug Combination Therapy Outcome Drug Comparisons LDL MET/RSG is associated with higher levels of LDL, by 14.5 mg/dL, when compared to MET. HDL When compared to MET monotherapy: • MET/RSG is associated with higher HDL levels by 2.8 mg/dL. • MET/DPP-4 inhibitors are associated with similar levels of HDL. • MET/Pio is associated with higher levels of HDL. TG MET is associated with lower TG levels, by 14.5 mg/dL, when compared to MET/RSG. Strength of Evidence High High Moderate Low High DPP-4 inhibitors = dipeptidyl peptidase-4 inhibitors; HbA1c = hemoglobin A1c; HDL = high-density lipoprotein; LDL = low-density lipoprotein; MET = metformin; Pio = pioglitazone; RSG = rosiglitazone; TG = triglycerides. Details of HbA1c and Weight Intermediate Outcomes: Comparisons of Two-Drug Combination Therapies Outcome Drug Comparisons HbA1c MET/SU and MET/TZD are associated with similar HbA1c levels. Weight MET/SU had a more favorable effect on weight when compared to these combination therapies: • TZD/SU, by -3.2 kg; MET/TZD, by -0.9 kg. MET/GLP-1 receptor agonists has a more favorable effect on weight, by about 1.9 to 12.3 kg, when compared to: • MET/SU, MET/TZD, MET/basal insulin, and MET/DPP-4 inhibitor. MET/DPP-4 inhibitors more favorably affect weight, by about 1.5 to 2.5 kg, when compared to • MET/TZD and MET/SU. Strength of Evidence Moderate Moderate Low Low DPP-4 inhibitors = dipeptidyl peptidase-4 inhibitors; GLP-1 receptor agonists = glucagon-like peptide-1 receptor agonists; HbA1c = hemoglobin A1c; MET = metformin; RSG = rosiglitazone; SU = second-generation sulfonylureas; TZD = thiazolidinediones. Details of Lipid Intermediate Outcomes: Comparisons of Two-Drug Combination Therapies Outcome Drug Comparisons LDL MET/SU is associated with lower levels of LDL, by -13.5 mg/dL, when compared with MET/RSG. HDL When compared with MET/SU: • MET/Pio is associated with higher HDL levels by 5 mg/dL. • MET/RSG is associated with higher levels of HDL by 2.7 mg/dL. • SU/Pio is associated with higher levels of HDL. TG Strength of Evidence Moderate Moderate Moderate Low When compared with MET/SU: • MET/Pio is associated with lower levels of TG, by -15 mg/dL. • MET/RSG is associated with similar levels of TG. HDL = high-density lipoprotein; LDL = low-density lipoprotein; MET = metformin; Pio = pioglitazone; RSG = rosiglitazone; SU = second-generation sulfonylureas; TG = triglycerides. Moderate Moderate Comparative Adverse Events and Side Effects Hypoglycemia Gastrointestinal side effects Liver injury Hip and nonhip fractures Congestive heart failure Severe lactic acidosis Cancer Severe allergic reactions Pancreatitis Cholecystitis Macular edema or decreased vision Overview of Adverse Events and Side Effects SUs and MEGs may cause more hypoglycemia than MET, TZDs, or DPP-4 inhibitors (moderate to high strength of evidence). Most MET combinations increased the risk of hypoglycemia (moderate strength of evidence). MET is associated with more GI adverse events (mainly diarrhea) when compared to other agents (moderate to high strength of evidence). TZDs are associated with a higher risk of CHF compared to SUs (moderate strength of evidence).* TZDs alone or in combination are associated with a higher risk of fractures compared to other agents (high strength of evidence). In 2007, the FDA issued an alert and changed labeling to state that TZDs cause or exacerbate CHF in some patients. In 2010, the FDA placed additional prescribing restrictions on rosiglitazone use for type 2 diabetes in response to data that suggest an elevated risk of cardiovascular events including myocardial infarction and stroke. CHF = congestive heart failure; DPP-4 inhibitors = dipeptidyl peptidase-4 inhibitors; GI = gastrointestinal; MEG = meglitinides; MET = metformin; SU = second-generation sulfonylureas; TZD = thiazolidinediones. Hypoglycemic Adverse Events: Comparisons of Monotherapies Outcome Mild to moderate hypoglycemia Drug Comparisons The risk of hypoglycemia for SUs was: • 4.6-fold higher than with MET • 3.9-fold higher than with TZDs • Higher than with DPP-4 inhibitors When compared with MET monotherapy: • MEGs were associated with a 3-fold increase in hypoglycemia • TZDs were associated with a similar risk of hypoglycemia Strength of Evidence High High Moderate Moderate Moderate DPP-4 inhibitors = dipeptidyl peptidase-4 inhibitors; MEG = meglitinides; MET = metformin; SU = second-generation sulfonylureas; TZD = thiazolidinediones. Gastrointestinal Adverse Events: Comparisons of Monotherapies Outcome GI adverse events Drug Comparisons MET was associated with more GI adverse events than were: •TZDs •SU; DPP-4 inhibitors TZDs and SUs were associated with similar rates of GI adverse events. Strength of Evidence High Moderate High DPP-4 inhibitors = dipeptidyl peptidase-4 inhibitors; GI = gastrointestinal; MET = metformin; SU = second-generation sulfonylureas; TZD = thiazolidinediones. Other Adverse Events and Side Effects: Comparisons of Monotherapies Outcome Liver injury Drug Comparisons Rates of liver injury for TZDs were low (range, 0% to 0.9%) and were similar to: • SUs (range 0% to 1%) • MET (range 0.8% to 2.2%) Hip/nonhip fractures TZDs were associated with higher rates of bone fractures compared with MET. CHF Rates of CHF are higher for TZDs than for: • SUs • MET Severe lactic acidosis While the risk of severe lactic acidosis was low for MET, SU, and MET/SU, studies excluded individuals with significant renal, liver, or cardiovascular disease. Strength of Evidence High Moderate High Moderate Low Moderate CHF = congestive heart failure; GI = gastrointestinal; MET = metformin; SU = second-generation sulfonylureas; TZD = thiazolidinediones. Adverse Events and Side Effects: Monotherapy Versus Two-Drug Combination Therapy Outcome Drug Comparisons Mild to moderate hypoglycemia When compared with MET monotherapy: • MET/SU is associated with an increased risk. • MET/TZD is associated with an increased risk. • MET/DPP-4 inhibitors are associated with a similar risk. GI adverse events MET is associated with more GI adverse events than these combinations if the dose of MET is higher in the monotherapy arm: • MET/SUs • MET/TZDs Hip/nonhip fractures MET/TZD is associated with higher rates of bone fractures than MET. Strength of Evidence Moderate Moderate Moderate Moderate Moderate High DPP-4 inhibitors = dipeptidyl peptidase-4 inhibitors; GI = gastrointestinal; MET = metformin; SU = second-generation sulfonylureas; TZD = thiazolidinediones. Adverse Events and Side Effects: Comparisons of Two-Drug Combination Therapies Outcome Mild to moderate hypoglycemia Drug Comparisons MET/SU is associated with higher levels of hypoglycemia than these combinations: • MET/TZDs • MET/GLP-1 receptor agonists • Modestly lower risk of hypoglycemia when MET is combined with a basal insulin rather than a premixed insulin. Strength of Evidence High Moderate Moderate GI adverse events MET/SU is associated with more GI adverse events than SU/TZD. Moderate Hip/nonhip fractures TZD combinations are associated with higher rates of bone fractures than MET/SU. High GI = gastrointestinal; GLP-1 receptor agonists = glucagon-like peptide-1 receptor agonists; MET = metformin; SU = second-generation sulfonylureas; TZD = thiazolidinediones. Macrovascular and Microvascular Long-Term Clinical Outcomes of Diabetes All-cause mortality Cardiovascular mortality Cardiovascular and cerebrovascular disease morbidity Retinopathy Nephropathy Neuropathy Long-Term Clinical Outcomes: Overview Metformin was associated with slightly lower all-cause mortality and cardiovascular disease mortality than were sulfonylureas (low strength of evidence). Pioglitazone was better than metformin at reducing the albumin-to-creatinine ratio, likely indicating less nephropathy (moderate strength of evidence). There was insufficient evidence to draw conclusions about other comparisons of antidiabetic medications. Comparative Effectiveness and Safety in Subpopulations Twenty-eight studies addressed medication effectiveness and safety in subpopulations. When compared to men, women taking RSG either as monotherapy or in combination were at higher risk for bone fractures than were those taking MET alone or in combination with SUs. However, for the majority of comparisons, the available studies did not have sufficient power to allow for subgroup analyses, and few studies occurred exclusively in a subpopulation. There was no conclusive information to predict which subgroups of patients might differentially respond to alternative treatments. MET = metformin; RSG = rosiglitazone; SU = second-generation sulfonylureas. Gaps in Knowledge Studies are needed to address the efficacy of treatments for: Patients with type 2 diabetes who have varying levels of underlying cardiovascular and renal disease. Persons of different ethnic groups or variant forms of type 2 diabetes. Additional comparative studies are needed including: Comparisons of newer medications. Combinations with basal or premixed insulin and MET or other antidiabetic agents. Additional two-drug combinations. Sufficient data on event rates are needed to analyze major clinically important outcomes, adverse events, and long-term complications of type 2 diabetes. What to Discuss With Your Patients Establishing a goal for HbA1c and strategies to help accomplish that goal, including weight loss and exercise along with consistent use of medication Strategies to increase adherence include creating a medication schedule, addressing the costs of medications, and reporting adverse events in a timely manner The need for regular glucose testing and routine blood tests for HbA1c