* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PowerPoint プレゼンテーション - 埼玉医科大学総合医療センター 内分泌
Survey
Document related concepts
Plateau principle wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Pharmacognosy wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Prescription costs wikipedia , lookup
Neuropharmacology wikipedia , lookup
Theralizumab wikipedia , lookup
Transcript
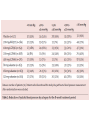
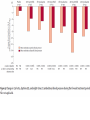
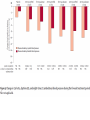
Journal Club Phung OJ, Scholle JM, Talwar M, Coleman CI Effect of Noninsulin Antidiabetic Drugs Added to Metformin Therapy on Glycemic Control, Weight Gain, and Hypoglycemia in Type 2 Diabetes JAMA. 2010;303(14):1410-1418 Ruilope LM, Dukat A, Böhm M, Lacourcière Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebocontrolled, active comparator study. Lancet. 2010 Apr 10;375(9722):1255-66. 2010年4月15日 8:30-8:55 8階 医局 埼玉医科大学 総合医療センター 内分泌・糖尿病内科 Department of Endocrinology and Diabetes, Saitama Medical Center, Saitama Medical University 松田 昌文 Matsuda, Masafumi Diabetes Care 32:193–203, 2009 University of Connecticut School of Pharmacy, Storrs, and Drug Information Center, Hartford Hospital, Hartford, Connecticut. JAMA. 2010;303(14):1410-1418 Aim Context Metformin is the recommended initial drug therapy for patients with type 2 diabetes mellitus (DM). However, the optimal secondline drug when metformin monotherapy fails is unclear. Objective To determine the comparative efficacy, risk of weight gain, and hypoyglycemia associated with noninsulin antidiabetic drugs in patients with type 2 DM not controlled by metformin alone. Methods Data Sources A literature search via MEDLINE (beginning in January 1950) and Cochrane CENTRAL through January 2010 and a manual search of references for additional relevant studies. Study Selection Randomized controlled trials (RCTs) with at least 3 months’ duration, evaluating noninsulin antidiabetic drugs added to metformin in patients experiencing an inadequate response to maximized and stable (_4 weeks at _1500 mg or maximally tolerated dose) metformin therapy. Data Extraction Inclusion/exclusion criteria; duration of patient follow-up; drug, dose, and schedule used; use of concurrent lifestyle modification; and baseline characteristics (age, sex, anthropometrics, glycated hemoglobin A1c [HbA1c], duration of DM, and metformin dose). End points collected included mean change in HbA1c, proportion of patients achieving HbA1c goal of less than 7%, change in weight, and incidence of hypoglycemia. Mixed-treatment comparison meta-analysis was used to calculate the weighted mean difference for changes from baseline in HbA1c and body weight and relative risk (RR) of HbA1c goal attainment and hypoglycemia, with associated 95% credible intervals. Figure. Flow Diagram of RCTs Evaluating the Use of Noninsulin Antidiabetic Drugs Added to Metformin in Patients With Type 2 Diabetes RCTs indicate randomized controlled trials. aProvided information about study design or patient demographics. ΔHbA1C Jadad Score: Oxford quality scoring system (highest quality to lowest) Was the study described as randomized? yes and good=2, yes but not good=1, no=0 Was the study described as double blind? yes and good=2, yes but not good=1, no=0 Was there a description of withdrawals and dropouts? yes =1, no=0 Results Data Synthesis Overall, 27 RCTs (n=11 198) were included. Mean (range) trial duration was 32 (12-52) weeks. The different classes of drugs were associated with similar HbA1c reductions (range, 0.64%0.97%) compared with placebo. Although use of thiazolidinediones, sulfonylureas, and glinides were associated with weight gain (range, 1.77-2.08 kg), glucagon-like peptide-1 analogs, α-glucosidase inhibitors, and dipeptidyl peptidase-4 inhibitors were associated with weight loss or no weight change. Sulfonylureas and glinides were associated with higher rates of hypoglycemia than with placebo (RR range, 4.57-7.50). Conclusion When added to maximal metformin therapy, all noninsulin antidiabetic drugs were associated with similar HbA1c reductions but differed in their associations with weight gain and risk of hypoglycemia. Message 欧米はメトホルミンから開始するが2番目の薬 物は? どれも同程度の血糖降下作用があるという?? (SUやGLP-1は低下するはずだが???) SU薬を1番目の薬物とした場合は? Division of Hypertension, Hospital 12 de Octubre, Madrid, Spain (Prof L M Ruilope MD); Second Department of Internal Medicine, Comenius University, Bratislava, Slovakia (Prof A Dukat MD); Department of Internal Medicine III, Cardiology, Angiology, and Intensive Care, Universitat des Saarlandes, Homburg/Saar, Germany (Prof M Bohm MD); Hypertension Research Unit, Centre Hospitalier de l’Universite Laval, QC, Canada (Y Lacourciere MD); and Novartis Pharmaceuticals, East Hanover, NJ, USA (J Gong PhD, M P Lefk owitz MD) This trial is registered with ClinicalTrials.gov, number NCT00549770. Lancet 2010; 375: 1255–66 Background LCZ696 is a first-in-class inhibitor of the angiotensin II receptor and neprilysin. We aimed to establish whether the dual actions of LCZ696 lead to further lowering of blood pressure, compared with the angiotensinreceptor blocker valsartan. Method 1328 patients aged 18–75 years with mild-tomoderate hypertension were randomly assigned (double-blind) to 8 weeks’ treatment in one of eight groups: 100 mg (n=156 patients), 200 mg (n=169), or 400 mg (n=172) LCZ696; 80 mg (n=163), 160 mg (n=166), or 320 mg (n=164) valsartan; 200 mg AHU377 (n=165); or placebo (n=173). The primary endpoint was the mean difference across the three single-dose pairwise comparisons of LCZ696 versus valsartan (100 mg vs 80 mg, 200 mg vs 160 mg, and 400 mg vs 320 mg) in mean sitting diastolic blood pressure during the 8-week treatment period. Analysis was by intention to treat. Data are mean (SD) or number of patients (%). *Data supplied for 170 patients on placebo, 164 on 200 mg AHU377, 154 on 100 mg LCZ696, 168 on 200 mg LCZ696, 172 on 400 mg LCZ696, 162 on 80 mg valsartan, 166 on 160 mg valsartan, and 162 on 320 mg valsartan; data were missing for remaining patients. †Data were recorded at study entry (week 0) apart from blood pressure measurements, which were recorded at baseline (week 4) just before patients were given the first dose of study drug. Systolic BP Diastolic BP Systolic BP Diastolic BP Results 1215 patients completed the 8-week treatment period. The average reduction in mean sitting diastolic blood pressure across the doses of LCZ696 versus the appropriate comparator dose of valsartan showed significantly greater reductions with LCZ696 (mean reduction: –2・17 mm Hg, 95% CI –3・28 to –1・06; p<0・ 0001). The reduction in mean sitting diastolic blood pressure was significantly different for 200 mg LCZ696 versus 160 mg valsartan (–2・97 mm Hg, 95% CI –4・88 to –1・07, p=0・0023) and for 400 mg LCZ696 versus 320 mg valsartan (–2・70 mm Hg, –4・61 to –0・80, p=0・0055). LCZ696 was well tolerated and no cases of angiooedema were reported; only three serious adverse events occurred during the 8-week treatment period, of which none was judged to be related to the study drug, and no patients died. Conclusion Compared with valsartan, dualacting LCZ696 provides complementary and fully additive reduction of blood pressure, which suggests that the drug holds promise for treatment of hypertension and cardiovascular disease. Message 1つの分子に2つの作用点のある降圧薬! 副作用軽減で有用性があるという。 合剤でもよいかもしれないが???