* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Drug Induced Liver Injury (DILI) - The University of North Carolina at
Orphan drug wikipedia , lookup
Compounding wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug interaction wikipedia , lookup
Neuropharmacology wikipedia , lookup
Wilson's disease wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Theralizumab wikipedia , lookup
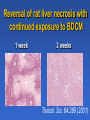
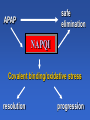
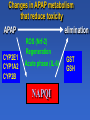
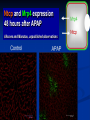
Drug Induced Liver Injury: Implications in drug discovery and development Paul B. Watkins University of North Carolina Chapel Hill, N.C. Drug Induced Liver Injury (DILI) is Hot FDA / Pharma steering committee Several Critical Path Initiatives $ millions spend in industry New Network (DILIN) SAE Consortium Industry SAE Priorities 2006 Rank Order [1 highest to 5 lowest] Overall Priority Variance Your Company's Priority Variance Hepatotoxicity 1.1 low 1.2 low QT Prolongation 2.6 moderate 2.5 high Rhabdomyolosis 3.3 moderate 3.5 mod Serious Skin Rashes [SJS] 3.5 high 3.4 high Edema 4.4 high 4.5 high SAE Consortium Survey – courtesy of Arthur Holden Troglitazone (Rezulin®) 1). PPARg agonist 2). Treats type 2 diabetes 3). Caused liver failure Presentation by Dr. Mark Pierce (Parke-Davis Pharmaceutical Research) Overall Post-Marketing Reporting Death/Transplant Rate March 1997 - March 1999 35 in 1.58 million = 1 in 45,098 Background incidence of liver failure with no known cause ~ 1 in 1 million March 26th 1999 - Troglitazone - FDA Advisory Panel Troglitazone (Rezulin®) 1). Acute liver failure reports continued despite warnings and monitoring recommendations 2). Second in class (2) came on the market and appeared to be safer 3). Withdrawn from the market Troglitazone (Rezulin®) The Rise and Fall of the Killer Drug Rezulin Los Angeles Times, June 4, 2000, p.1A. “…a disparate collection of physicians inside the U.S. Food and Drug Administration waged a remarkable revolt that … combined meticulous research and bluntly worded e-mail messages to upbraid their government superiors for contributing to the needless deaths of patients.” 45 40 35 30 25 20 15 10 5 0 Un kn ow n/ ot he r fg oo ds Co st o er cia l Co m m Ef f ic ac y Series1 Series1 Series2 PK /b io av ai la Sa bi lit fe y ty /T ox ic ol og y % of total terminations Why clinical drug development programs were terminated in 1991 Nature Reviews: Drug Discovery, Aug, 2004 45 40 35 30 25 20 15 10 5 0 Series1 1991 Un kn ow n/ ot he r fg oo ds Co st o er cia l Co m m Ef f ic ac y Series2 2000 PK /b io av ai la Sa bi lit fe y ty /T ox ic ol og y % of total terminations Why clinical drug development programs were terminated in 2000 Nature Reviews: Drug Discovery, Aug, 2004 “Hepatotoxicity has been the most common single adverse effect causing major drug problems, including withdrawals and refusals to approve” Bob Temple, M.D. FDA 2/15/01 2006 State of the Art How to avoid hepatotoxicity in drug development 1). Avoid certain molecular structures Compound Pair O Ibuprofen “Clean” Compound HO O Ibufenac “Toxic” Compound* HO *withdrawn from the market in the 1960’s because of clinical liver toxicity 2006 State of the Art How to avoid hepatotoxicity in drug development 1). Avoid certain molecular structures 2). Target daily dose to < 10 mg/day 2006 State of the Art How to avoid hepatotoxicity in drug development 1). Avoid certain molecular structures 2). Target daily dose to < 10 mg/day 3). Low covalent binding in liver microsomes 4). Low production of glutathione conjugates 2006 State of the Art How to avoid hepatotoxicity in drug development 1). Avoid certain molecular structures 2). Target daily dose to < 10 mg/day 3). Low covalent binding in liver microsomes 4). Low production of glutathione conjugates 5). Low incidence (<5%) of ALT > 3 X ULN in clinical trials. Example: Acetaminophen 1). Avoid certain molecular structures - NO 2). Target daily dose to < 10 mg/day – 4 grams/day 3). Low covalent binding in liver microsomes – NO 4). Low production of glutathione conjugates – NO 5). Low incidence (<5%) of ALT - NO Watkins et al. ALT vs. time for APAP Group (n = 26), ULN = 40 Solid Line = on treatment, Dotted Line = off treatment 640 16 X APAP Mean ALT 3 X ULN R eference 14 X 480 12 X 400 10 X 320 8X 240 6X 160 4X 80 2X 0 Fold ULN for ALT ALT 560 0X 0 5 10 15 Time (days) 20 25 30 JAMA, 392:87,2006 Example: Acetaminophen 1). Avoid certain molecular structures - NO 2). Target daily dose to < 10 mg/day – 4 grams/day 3). Low covalent binding in liver microsomes – NO 4). Low production of glutathione conjugates – NO 5). Low incidence (<5%) of ALT - NO Drug Induced Liver Injury (DILI) 1). Thorn in the side of drug development. 2). High priority to design out of drugs. 3). Little progress made to date. Drug Induced Liver Injury (DILI) can mimick every known liver disease Cholestasis (&vanishing bile duct syndrome) Steatosis (micro and macrovesicular) Phospholipidosis Veno-occlusive disease Occult fibrosis/ cirrhosis Liver cancer Acute hepatocellular injury – High ALT/AST Regulatory actions due to DILI (1995-2006) Withdrawals bromfenac troglitazone pemoline Second Line felbamate tolcapone trovafloxacin http://www.fda.gov/medwatch/safety.htm Warnings acetaminophen leflunomide nefazodone nevirapine pyrazinamide/rifampin terbinafine valproic acid zifirlukast atomoxetine interferon 1b –1b and 1a saquinavir infliximab bosentan telithromycin (kava, lipokinex) Regulatory actions due to DILI (1995-2006) Withdrawals bromfenac troglitazone pemoline Second Line felbamate tolcapone trovafloxacin http://www.fda.gov/medwatch/safety.htm Warnings acetaminophen leflunomide nefazodone nevirapine pyrazinamide/rifampin terbinafine valproic acid zifirlukast atomoxetine interferon 1b –1b and 1a saquinavir infliximab bosentan telithromycin (kava, lipokinex) Of the 23 drugs/CAM that have undergone withdrawal, restriction or warnings 19/23 (82%) were associated with acute idiosyncratic hepatocellular injury “idiosyncracy” (Hippocrates, ~400 B.C.) (idios) - one’s own, self (syn) - together (crasis) - a mixing, mixture therefore a person’s own mixture of characteristics, factors, nature and nurture, uniquely John Senior - FDA Liver injury ( ALT) safe SAFE Concept of idiosyncratic hepatocellular injury death jaundice enceph Days on drug Challenges in identifying factors underlying susceptibility to DILI 1). How to identify susceptible individuals. 2). What to do with them once you have them. Liver injury ( ALT) safe SAFE Concept of idiosyncratic hepatocellular injury Selection of patients based on serial ALT values in a clinical trial 1.34 C.V. CV ALT Non-susceptible cases 0.34 susceptible 0.23 0.16 controls 0.03 0.18 0.58 0.77 1.10 34.77 ULN Maximum ALT, fold Upper Limit of Normal (logarithmic scale) A genetic test that predicts ALT elevations: 1). Would obviate need for ALT monitoring. 2). Would be useful in developing next in class drugs. 3). May provide only limited insight into mechanisms of idiosyncratic severe DILI. Problem with ALT elevation as the endpoint 1). Occurs with drugs that do not have clinically important liver toxicity 2). Usually reverse with continued treatment even with drugs that can cause acute liver failure. Incidence of ALT elevations (>3X ULN) and clinical hepatitis ALT hepatitis troglitazone 2% INH 15% diclofenac 3% <0.1 <1% <0.01% Treatment with tacrine through ALT elevations unpublished Reversed on treatment Treatment stopped unpublished Reversal of rat liver necrosis with continued exposure to BDCM 1 week 3 weeks Toxicol. Sci. 64:268 (2001) Possible explanations for reversibility of ALT elevations 1). ALT elevations that reverse on treatment have no relationship to those that can progress to liver failure. 2). A subset of those with ALT elevations can progress on to liver failure (i.e. those who can not adapt). Safe pathways Drug elimination Reactive Metabolite X ALT elevations Adaptation Progressive injury jaundice liver failure Increased ALT safe SAFE Concept of idiosyncratic hepatocellular injury safe elimination APAP NAPQI Covalent binding/oxidative stress resolution progression Effect of 8 days APAP pretreatment (---) on single dose toxicity in mice Hepatology 29:436, 1999. 3-Cys-APAP adducts (brown) 2 hours after single toxic APAP dose Saline pretreatment APAP pretreatment Changes in APAP metabolism that reduce toxicity APAP elimination CYP2E1 CYP1A2 CYP2B ROS (Nrf-2) Regeneration Acute phase (IL-6) NAPQI GST GSH Transporters during recovery from APAP hepatotoxicity TNFa X X Transporters during recovery from APAP hepatotoxicity Ntcp and Mrp4 expression 48 hours after APAP Alkunes and Manatou, unpublished observations MDR1 (P-glycoprotein) expression MDR1 In submassive necrosis (human) Normal liver necrosis J Pathol 200:553, 2003 MRP3 expression in submassive necrosis (human) Normal liver MRP3 necrosis J Pathol 200:553, 2003 Conclusion Adaptation to liver toxicity can involve: a). Down regulation of CYPs and uptake transporters b). Upregulation of glutathione and efflux transporters 140 120 100 80 1st study 1st study 2nd study Day ffool llloow w-uupp 1133 1 1 9 7 5 3 60 40 20 0 1 ALT (U/L) Serial ALT in healthy woman receiving APAP 1 g qid X 7 days Unpublished data Conclusions • It is adapation to toxicity. • Arguably the most important issue in idiosyncrasy. – a). Determines whether patient gets sick – b). Implications for monitoring – b). Susceptibilities may not be drug specific • Current concepts do not account for the “memory”. Where do we go from here? The most appropriate model for studying idiosyncratic hepatotoxicity are the people who actually experienced this. Selection of cases and controls from serial ALT values in a clinical trial 1.34 C.V. CV ALT Non-susceptible cases 0.34 susceptible 0.23 0.16 controls 0.03 0.18 0.58 0.77 1.10 34.77 ULN Maximum ALT, fold Upper Limit of Normal (logarithmic scale) A cooperative agreement funded by the Division of Digestive Diseases and Nutrition, National Institute of Diabetes and Digestive and Kidney Diseases http://dilin.dcri.duke.edu/ Sphere of Influence 12.8 million lives http://dilin.dcri.duke.edu/ Resources Created by DILIN 1). Genomic DNA bank. 2). Immortalized lymphocyte bank. 3). Registry of subjects. Final take home points 1). The DILIN network represents the best opportunity to date to identify mechanisms underlying severe idiosyncratic DILI. 2). Research utilizing the resulting resources will be challenging. Idiosyncratic hepatocellular injury due to drugs is a model for all environmental disease 1). Large population with known “exposure” to a defined xenobiotic (the drug). 2). Biomarkers that are cheap, safe, and sensitive.