* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Antimicrobial Agents
Drug design wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Drug discovery wikipedia , lookup
Prescription costs wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Psychopharmacology wikipedia , lookup
Theralizumab wikipedia , lookup
Levofloxacin wikipedia , lookup
Drug interaction wikipedia , lookup
Ciprofloxacin wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
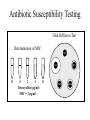
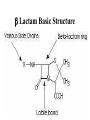
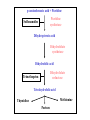
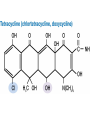
Antimicrobial Agents Martin Votava Olga Kroftová Overview • If bacteria make it past our immune system and start reproducing inside our bodies, they cause disease. • Certain bacteria produce chemicals that damage or disable parts of our bodies. • Antibiotics work to kill bacteria.Antibiotics are specific to certain bacteria and disrupt their function. What is an Antibiotic? • An antibiotic is a selective poison. • It has been chosen so that it will kill the desired bacteria, but not the cells in your body. Each different type of antibiotic affects different bacteria in different ways. • For example, an antibiotic might inhibit a bacteria's ability to turn glucose into energy, or the bacteria's ability to construct its cell wall. Therefore the bacteria dies instead of reproducing. Antibiotics • Substances produced by various species of microorganisms: bacteria, fungi, actinomycetes- to suppress the growth of other microorganisms and to destroy them. Today the term ATB extends to include synthetic antibacterial agents: sulfonamides and quinolones. History • The German chemist Paul Ehrlich developed the idea of selective toxicity: that certain chemicals that would be toxic to some organisms, e.g., infectious bacteria, would be harmless to other organisms, e.g., humans. • In 1928, Sir Alexander Fleming, a Scottish biologist, observed that Penicillium notatum, a common mold, had destroyed staphylococcus bacteria in culture. Sir Alexander Fleming Fleming’s Petri Dish Zone of Inhibition • Around the fungal colony is a clear zone where no bacteria are growing • Zone of inhibition due to the diffusion of a substance with antibiotic properties from the fungus History • Penicillin was isolated in 1939, and in 1944 Selman Waksman and Albert Schatz, American microbiologists, isolated streptomycin and a number of other antibiotics from Streptomyces griseus. Susceptibility vs. Resistance of microorganisms to Antimicrobial Agents • Success of therapeutic outcome depends on: • Achieving concentration of ATB at the site of infection that is sufficient to inhibit bacterial growth. • Host defenses maximally effective –MI effect is sufficient – bacteriostatic agents (slow protein synthesis, prevent bacterial division) • Host defenses impaired- bactericidal agents • Complete ATB-mediated killing is necessary Susceptibility vs. Resistance (cont.) • Dose of drug has to be sufficient to produce effect inhibit or kill the microorganism: • However concentration of the drug must remain below those that are toxic to human cells – • If can be achieved – microorganism susceptible to the ATB • If effective concentration is higher than toxicmicroorganism is resistant Susceptibility vs. Resistance (cont.) • Limitation of in vitro tests • In vitro sensitivity tests are based on non-toxic plasma concentrations –cut off • Do not reflect concentration at the site of infection • E.g.: G- aer.bacilli like Ps.aeruginosa inhibited by 2 – 4 ug/ml of gentamycin or tobramycin. Susceptible !? Antibiotic Susceptibility Testing Disk Diffusion Test Determination of MIC Str Tet 8 4 2 1 Tetracycline (μg/ml) MIC = 2 μg/ml 0 Ery Chl Amp Susceptibility vs. Resistance (cont.) • Plasma concentration above 6-10 ug/ml may result in ototoxicity or nephrotoxicity • Ration of toxic to therapeutic concentration is very low –agents difficult to use. • Concentration in certain compartments – vitreous fluid or cerebrospinal fluid much lower than those in plasma. • Therefore can be only marginally effective or ineffective even those in vitro test states sensitive. Susceptibility vs. Resistance (cont.) • Therefore can be only marginally effective or ineffective even those in vitro test states „sensitive“. • Conversely – concentration of drug in urine may be much higher than in plasma , so „resistant“ agents can be effective in infection limited to urine tract Resistance • To be effective ATB must reach the target and bind to it. • Resistance: • Failure to reach the target • The drug is inactivated • The target is altered Resistance (cont.) • Bacteria produce enzymes at or within the cell surface –inactivate drug • Bacteria possess impermeable cell membrane prevent influx of drug. • Transport mechanism for certain drug is energy dependent- not effective in anaerobic environment. • ATB as organic acids penetration is pH – dependent. Resistance (cont.) • Acquired by mutation and passed vertically by selection to daughter cells. • More commonly – horizontal transfer of resistance determinant from donor cell, often another bacterial species, by transformation, transduction, or conjugation. • Horizontal transfer can be rapidly disseminated • By clonal spread or resistant strain itself • Or genetic exchange between resistant and further susceptible strains. Resistance (cont.) • Methicilin resistant strains of Staphylococcus aureus clonally derived from few ancestral strains with mecA gene • Encodes low-affinity penicillin-binding protein that confers methicillin resistance. • Staphylococcal beta-lactamase gene, which is plasmid encoded, presumambly transferred on numerous occasions. Because is widely distributed among unrelated strains, identified also in enterococci Selection of the ATB • Requires clinical judgment, detailed knowledge of pharmacological and microbiological factors. • Empirical therapy – initial – infecting organism not identified – single broad spectrum agent • Definitive therapy- microorganism identified – a narrow –spectrum low toxicity regiment to complete the course of treatment Empirical and Definite Therapy • Knowledge of the most likely infecting microorganism and its susceptibility • Gram stain • Pending isolation and identification of the pathogen • Specimen for culture from site of infection should be obtain before initiation of therapy • Definite therapy Penicillins • Penicillins contain a b-lactam ring which inhibits the formation of peptidoglycan crosslinks in bacterial cell walls (especially in Gram-possitive organisms) • Penicillins are bactericidal but can act only on dividing cells • They are not toxic to animal cells which have no cell wall Synthesis of Penicillin b-Lactams produced by fungi, some ascomycetes, and several actinomycete bacteria b-Lactams are synthesized from amino acids valine and cysteine b Lactam Basic Structure Penicillins (cont.) Clinical Pharmacokinetics • Penicillins are poorly lipid soluble and do not cross the blood-brain barrier in appreciable concentrations unless it is inflamed (so they are effective in meningitis) • They are actively excreted unchanged by the kidney, but the dose should be reduced in severe renal failure Penicillins (cont.) Resistance • This is the result of production of b-lactamase in the bacteria which destroys the b-lactam ring • It occurs in e.g. Staphylococcus aureus, Haemophilus influenzae and Neisseria gonorrhoea Penicillins (cont.) Examples • There are now a wide variety of penicillins, which may be acid labile (i.e. broken down by the stomach acid and so inactive when given orally) or acid stable, or may be narrow or broad spectrum in action Penicillins (cont.) Examples • Benzylpenicillin (Penicillin G) is acid labile and b-lactamase sensitive and is given only parenterally • It is the most potent penicillin but has a relatively narrow spectrum covering Strepptococcus pyogenes, S. pneumoniae, Neisseria meningitis or N. gonorrhoeae, treponemes, Listeria, Actinomycetes, Clostridia Penicillins (cont.) Examples • Phenoxymethylpenicillin (Penicillin V) is acid stable and is given orally for minor infections • it is otherwise similar to benzylpenicillin Penicillins (cont.) Examples • Ampicillin is less active than benzylpenicillin against Gram-possitive bacteria but has a wider spectrum including (in addition in those above) Strept. faecalis, Haemophilus influenza, and some E. coli, Klebsiella and Proteus strains • It is acid stable, is given orally or parenterally, but is b-laclamase sensitive Penicillins (cont.) Examples • Amoxycillin is similar but better absorbed orally • It is sometimes combined with clavulanic acid, which is a b-lactam with little antibacterial effect but which binds strongly to b-lactamase and blocks the action of blactamase in this way • It extends the spectrum of amoxycillin Penicillins (cont.) Examples • Flucloxacillin is acid stable and is given orally or parenterally • It is b-lactamase resistant • It is used as a narrow spectrum drug for Staphylococcus aureus infections Penicillins (cont.) Examples • Azlocillin is acid labile and is only used parenterally • It is b-lactamase sensitive and has a broad spectrum, which includes Pseudomonas aeruginosa and Proteus species • It is used intravenously for life-threatening infections,i.e. in immunocompromised patients together with an aminoglycoside Penicillins (cont.) Adverse effects • Allergy (in 0.7% to 1.0% patients). Patient should be always asked about a history of previous exposure and adverse effects • Superinfections(e.g.caused by Candida ) • Diarrhoea : especially with ampicillin, less common with amoxycillin • Rare: haemolysis, nephritis Penicillins (cont.) Drug interactions • The use of ampicillin (or other broadspectrum antibiotics) may decrease the effectiveness of oral conraceptives by diminishing enterohepatic circulation Antistaphylococcus penicillins • Oxacillin, cloxacillin – Resistant against staphylococcus penicillinasis Cephalosporins • They also owe their activity to b-lactam ring and are bactericidal. • Good alternatives to penicillins when a broad -spectrum drug is required • should not be used as first choice unless the organism is known to be sensitive Cephalosporins • BACTERICIDAL- modify cell wall synthesis • CLASSIFICATION- first generation are early compounds • Second generation- resistant to β-lactamases • Third generation- resistant to β-lactamases & increased spectrum of activity • Fourth generation- increased spectrum of activity Cephalosporins • FIRST GENERATION- eg cefadroxil, cefalexin, Cefadrine - most active vs gram +ve cocci. An alternative to penicillins for staph and strep infections; useful in UTIs • SECOND GENERATION- eg cefaclor and cefuroxime. Active vs enerobacteriaceae eg E. coli, Klebsiella spp,proteus spp. May be active vs H influenzae and N meningtidis Cephalosporins • THIRD GENERATION- eg cefixime and other I.V.s cefotaxime,ceftriaxone,ceftazidine. Very broad spectrum of activity inc gram -ve rods, less activity vs gram +ve organisms. • FOURTH GENERATION- cefpirome better vs gram +ve than 3rd generation. Also better vs gram -ve esp enterobacteriaceae & pseudomonas aerugenosa. I.V. route only Cephalosporins (cont.) Adverse effects • Allergy (10-20% of patients wit penicillin allergy are also allergic to cephalosporins) • Nephritis and acute renal failure • Superinfections • Gastrointestinal upsets when given orally Aminoglycosides (bactericidal) streptomycin, kanamycin, gentamicin, tobramycin, amikacin, netilmicin, neomycin (topical) • Mode of action - The aminoglycosides irreversibly bind to the 16S ribosomal RNA and freeze the 30S initiation complex (30S-mRNAtRNA) so that no further initiation can occur. They also slow down protein synthesis that has already initiated and induce misreading of the mRNA. By binding to the 16 S r-RNA the aminoglycosides increase the affinity of the A site for t-RNA regardless of the anticodon specificity. May also destabilize bacterial membranes. • Spectrum of Activity -Many gram-negative and some gram-positive bacteria • Resistance - Common • Synergy - The aminoglycosides synergize with β-lactam antibiotics. The β-lactams inhibit cell wall synthesis and thereby increase the permeability of the aminoglycosides. Aminoglycosides Clinical pharmacokinetics • These are poorly lipid soluble and, therefore, not absorbed orally • Parenteral administration is required for systemic effect. • They do not enter the CNS even when the meninges are inflamed. • They are not metabolized. Aminoglycosides (cont.) Clinical pharmacokinetics • They are excreted unchanged by the kidney (where high concentration may occur, perhaps causing toxic tubular demage) by glomerular filtration (no active secretion). • Their clearance is markedly reduced in renal impairment and toxic concentrations are more likely. Aminoglycosides (cont.) Resistance • Resistance results from bacterial enzymes which break down aminoglycosides or to their decreased transport into the cells. Aminoglycosides (cont.) Examples • Gentamicin is the most commonly used, covering Gram-negative aerobes, e.g. Enteric organisms (E.coli, Klebsiella, S. faecalis, Pseudomonas and Proteus spp.) • It is also used in antibiotic combination against Staphylococcus aureus. • It is not active against aerobic Streptococci. Aminoglycosides (cont.) Examples • In addition to treating known sensitive organisms, it is used often blindly with other antibiotics in severe infections of unknown cause. • Streptomycin was formerly the mainstay of antituberculous therapy but is now rarely used in the developed world. Aminoglycosides (cont.) Examples • Tobramycin: used for pseudomonas and for some gentamicin-resistant organisms. • Some aminoglycosides,e.g. Gentamicin, may also be applied topically for local effect, e.g. In ear and eye ointments. • Neomycin is used orally for decontamination of GI tract. Aminoglycosides (cont.) Adverse effects • Although effective, aminoglycosides are toxic, and this is plasma concentration related. • It is essential to monitor plasma concentrations ( shortly before and after administration of a dose) to ensure adequate concentrations for bactericidal effects, while minimising adverse effects, every 2-3 days. Aminoglycosides (cont.) Adverse effects • The main adverse effects are: Nephrotoxicity Toxic to the 8th cranial nerve (ototoxic), especially the vestibular division. • Other adverse effects are not dose related, and are relatively rare, e.g. Allergies, eosinophilia. Macrolides (bacteriostatic) erythromycin, clarithromycin, azithromycin, spiramycin • Mode of action - The macrolides inhibit translocation by binding to 50 S ribosomal subunit • Spectrum of activity - Gram-positive bacteria, Mycoplasma, Legionella (intracellular bacterias) • Resistance - Common Macrolides (cont.) Examples and clinical pharmacokinetics • Erythromycin is acid labile but is given as an enterically coated tablet • Absorption is erratic and poor. • It is excreted unchanged in bile and is reabsorbed lower down the gastrointestinal tract (enterohepatic circulation). • It may be given orally or parenterally Macrolides (cont.) Examples and clinical pharmacokinetics • Macrolides are widely distributed in the body except to the brain and cerebrospinal fluid • The spectrum includes Staphylococcus aureus, Streptococcuss pyogenes, S. pneumoniae, Mycoplasma pneumoniae and Chlamydia infections. Macrolides (cont.) Examples and clinical pharmacokinetics • Newer macrolides such as clarithromycin and azithromycin may have fewer adverse effects. Macrolides – side effects • Nauzea, vomitus • Allergy • Hepatitis, ototoxicity • Interaction with cytochrome P450 3A4 (inhibition) Chloramphenicol, Lincomycin, Clindamycin (bacteriostatic) • Mode of action - These antimicrobials bind to the 50S ribosome and inhibit peptidyl transferase activity. • Spectrum of activity - Chloramphenicol - Broad range; Lincomycin and clindamycin - Restricted range • Resistance - Common • Adverse effects - Chloramphenicol is toxic (bone marrow suppression) but is used in the treatment of bacterial meningitis. Clindamycin • Clindamycin, although chemically distinct, is similar to erythromycin in mode of action and spectrum. • It is rapidly absorbed and penetrates most tissues well, except CNS. • It is particularly useful systematically for S. aureus (e.g.osteomyelitis as it penetrates bone well) and anaerobic infections. Clindamycin Adverse effects • Diarrhoea is common. • Superinfection with a strain of Clostridium difficile which causes serious inflammation of the large bowel (Pseudomembranous colitis) Chloramphenicol • This inhibits bacterial protein synthesis. • It is well absorbed and widely distributed , including to the CNS. • It is metabolized by glucoronidation in the liver. • Although an effective broad-spectrum antibiotics, its uses are limitid by its serious toxicity. Chloramphenicol (cont.) • The major indication is to treat bacterial meningitis caused by Haemophilus influenzae, or to Neisseria menigitidis or if organism is unknown.It is also specially used for Rikettsia (typhus). Chloramphenicol (cont.) Adverse effects • A rare anemia, probably immunological in origin but often fatal • Reversible bone marrow depression caused by its effect on protein synthesis in humans • Liver enzyme inhibition Sulfonamides and trimethoprim • Sulfonamides are rarely used alone today. • Trimethoprim is not chemically related but is considered here because their modes of action are complementary. Sulfonamides, Sulfones (bacteriostatic) • Mode of action - These antimicrobials are analogues of para-aminobenzoic acid and competitively inhibit formation of dihydropteroic acid. • Spectrum of activity - Broad range activity against grampositive and gram-negative bacteria; used primarily in urinary tract and Nocardia infections. • Resistance - Common • Combination therapy - The sulfonamides are used in combination with trimethoprim; this combination blocks two distinct steps in folic acid metabolism and prevents the emergence of resistant strains. Trimethoprim, Methotrexate, (bacteriostatic) • Mode of action - These antimicrobials binds to dihydrofolate reductase and inhibit formation of tetrahydrofolic acid. • Spectrum of activity - Broad range activity against grampositive and gram-negative bacteria; used primarily in urinary tract and Nocardia infections. • Resistance - Common • Combination therapy - These antimicrobials are used in combination with the sulfonamides; this combination blocks two distinct steps in folic acid metabolism and prevents the emergence of resistant strains. p-aminobenzoic acid + Pteridine Pteridine synthetase Sulfonamides Dihydropteroic acid Dihydrofolate synthetase Dihydrofolic acid Dihydrofolate reductase Trimethoprim Tetrahydrofolic acid Methionine Thymidine Purines Sulfonamides and trimethoprim Mode of action • Folate is metabolized by enzyme dihydrofolate reductase to the active tetrahydrofolic acid. • Trimethoprim inhibits this enzyme in bacteria and to a lesser degree in animal s, as the animal enzyme is far less sensitive than that in bacteria. Sulfonamides and trimethoprim Clinical pharmacokinetics • Most sulfonamides are well absorbed orally and they are widely distributed including to the CNS. • Most are excreted by the kidney unchanged. • They are effective against Gram-positive and many Gram-negative organism but are rarely used alone now. Sulfonamides and trimethoprim Clinical pharmacokinetics • Trimethoprim is also well absorbed and excreted by the kidneys, with similar spectrum. • Cotrimoxazole is widely used for urinary and upper respiratory tract infections but should not be the drug of choice because of its adverse effects. Sulfonamides and trimethoprim Clinical pharmacokinetics • It is the drug of choice for the treatment and prevention of pneumonia caused by Pneumocystis carinii in immunosupressed patients. • Trimethoprim is increasingly used alone for urinary tract and upper respiratory tract infections, as it is less toxic than the combination and equally effective. Sulfonamides and trimethoprim Adverse effects • Gastrointestinal upsets • Less common but more serious: -sulfonamides: allergy, rash, fever, agranulocytosis, renal toxicity -trimethoprim: macrocytis anemia, thrombocytopenia -cotrimoxazole: aplastic anemia Sulfonamides and trimethoprim Drug intereactions • Sulfonamides can decrease metabolism of phenytoin, warfarin and some oral hypoglycaemics, increasing their effects. Quinolones (bactericidal) nalidixic acid, ciprofloxacin, ofloxacin, norfloxacin, levofloxacin, lomefloxacin, sparfloxacin • Mode of action - These antimicrobials bind to the A subunit of DNA gyrase (topoisomerase) and prevent supercoiling of DNA, thereby inhibiting DNA synthesis. • Spectrum of activity - Gram-positive cocci and urinary tract infections • Resistance - Common for nalidixic acid; developing for ciprofloxacin Quinolones • The quinolones are effective but expensive antibiotics. • With increased use, resistance to these drugs is becoming more common. • They should in general be reverse drugs and not first-line treatment. Quinolones (cont.) Examples and clinical pharmacokinetics • Nalidixic acid, the first quinolone, is used as a urinary antiseptic and for lower urinary tract infections, as it has no systemic antibacterial effect. • Ciprofloxacin is a fluoroquinolone with a broad spectrum against Gram-negative bacilli and Pseudomonas, Quinolones (cont.) Examples and clinical pharmacokinetics • It can be given orally or i.v. to treat a wide range of infections, including respiratory and urinary tract infections as well as more serious infections, such as peritonitis and Salmonella. • Activity against anaerobic organism is poor and it should not be first choice for respiratory tract infections. Quinolones (cont.) Adverse effects • Gastrointestinal upsets • Fluoroquinolones may block the inhibitory neurotransmitter GABA, and this may cause confusion in the elderly and lower the fitting threshold. • They are also contraindicated in epileptics. • Allergy and anaphylaxis Quinolones (cont.) Adverse effects • Possibly damage to growing cartilage: not recommended for pregnant women and children Drug interaction • Ciprofloxacin is a liver enzyme inhibitor and may cause life-threatening interaction with theophylline. Tetracyclines (bacteriostatic) tetracycline, minocycline and doxycycline • Mode of action - The tetracyclines reversibly bind to the 30S ribosome and inhibit binding of aminoacyl-t-RNA to the acceptor site on the 70S ribosome. • Spectrum of activity - Broad spectrum; Useful against intracellular bacteria • Resistance - Common • Adverse effects - Destruction of normal intestinal flora resulting in increased secondary infections; staining and impairment of the structure of bone and teeth. Tetracyclines (cont.) Examples and clinical pharmacokinetics • Tetracycline, oxytetracycline have short half-lives. • Doxycycline has a longer half-life and can be given once per day. • These drugs are only portly absorbed. • They bind avidly to heavy metal ions and so absorption is greatly reduced if taken with food, milk, antacids or iron tablets. Tetracyclines (cont.) Examples and clinical pharmacokinetics • They should be taken at least half an hour before food. • Tetracyclines concentrate in bones and teeth. • They are excreted mostly in urine, partly in bile. • They are broad spectrum antibiotics, active against most bacteria except Proteus or Pseudomonas. Tetracyclines (cont.) Examples and clinical pharmacokinetics • Resistance is frequent. • They are specially indicated for Mycoplasma, Rikettsia, Chlamydia and Brucella infections. • Their most common use today is for acne, given either orally or topically. Tetracyclines (cont.) Adverse effects • Gastrointestinal upsets • Superinfection • Discolouration and deformity in growing teeth and bones (contraindicated in pregnancy and in children < 12 years) • Renal impairment (should be also avoided in renal disease) Metronidazole • Metronidazole binds to DNA and blocks replication. Pharmacokinetics • It is well absorbed after oral or rectal administration and can be also given i.v. • It is widely distributed in the body (including into abscess cavities) • It is metabolized by the liver. Metronidazole (cont.) Uses • Metronidazole is active against anaerobic organisms (e.g. Bacteroides, Clostridia), which are encountered particularly in abdominal surgery. • It is also used against Trichomonas, Giardia and Entamoeba infections and can be used to treat pseudomembranous colitis. Metronidazole (cont.) Uses • Increasingly, it is used as part of treatment of Helicobacter pyloris infestion of the stomach and duodenum associated with peptic ulcer disease. • It is used also to treat a variety of dental infections, particularly dental abscess. Metronidazole (cont.) Adverse effects • Nausea, anorexia and metallic taste • Ataxia • In patients, who drink alcohol, may occur unpleasant reactions. They should be advised not to drink alcohol during a treatment. • Possibly teratogenic if taken in the first trimester of pregnancy Nitrofurantoin • This is used as a urinary antiseptic and to treat Gram-negative infections in the lower urinary tract. • It is taken orally and is well absorbed and is excreted unchanged in the urine. • It only exerts its antimicrobial effect when it is concentrated in the urine and so has no systemic antibacterial effect. Nitrofurantoin (cont.) • It is ineffective in renal failure because of failure to concentrate. • Resistance develops relatively quickly. Nitrofurantoin (cont.) Adverse effects • Gastrointestinal upsets • Allergy • Polyneuritis Fucidin • Fucidin is active only against Staphylococcus aureus (by inhibiting bacterial protein synthesis) and is not affected b-lactamase. • It is usually only used with flucloxacillin to reduce the development of resistance. • It is well absorbed and widely distributed, including to bone Fucidin (cont.) • It can be given orally or parenterally. • It is metabolized in the liver. Adverse effects • Gastrointestinal upsets • Hepatitis and jaundice Vancomycin • This interferes with bacterial cell wall formation and is not absorbed after oral administration and must be given parenterally. • It is excreted by the kidney. • It is used i.v. to treat serious or resistant Staph. aureus infections and for prophylaxis of endocarditis in penicillin-allergic people. Vancomycin (cont.) • It is given orally to treat pseudomembranous colitis • teicoplanin is similar but less toxic Vancomycin (cont.) Adverse effects • Its toxicity is similar to aminoglycoside and likewise monitoring of plasma concentrations is essential. • Nephrotoxicity • Ototoxicity • Allergy Antibiotics for leprosy • Leprosy is caused by infection with Mycobacteria leprae. • A mixture of drugs are used to treat leprosy, depending on the type and severity of the infection and the local resistance patterns. Antibiotics for leprosy • Rifampicin is used and dapsone, which is related to the sulphoamides. • Its adverse effects include haemolysis, gastrointestinal upsets and rashes. Chemotherapy for viruses Antiviral drugs • Antiviral chemotherapy is still in its infancy. • Viruses are more difficult ‘targets’ than bacteria: they are most vulnerable during reproduction, but all use host cell organelles and enzymes to do this, so that antiviral compounds are often as toxic to host cells as to virus. Antiviral drugs (cont.) • Viruses have assumed increasing importance in the setting of immunosuppression - both drug induced and AIDS. Antiviral drugs (cont.) • Current antiviral drugs are thought to work in one of the following ways: - inhibition of viral ‘uncoating’ shortly after penetration into the cell; they are best for prophylaxis or very early in the disease course (e.g.amantadine) - interference with viral RNA synthesis and function (e.g. ribavirin) Antiviral drugs (cont.) – interference with DNA synthesis (e.g. cytarabine) – inhibition of viral DNA polymerase (e.g.aciclovir and gancyclovir) – inhibition of reverse transcriptase at retroviruses such as HIV (e.g.zidovudine) – use of complex natural antiviral defences by employing interferon Aciclovir Mode of action • It is active against Herpes simplex and Herpes zoster. • Aciclovir targets virus-infected cells quite specifically, and this explains the drug`s relatively low toxicity. Aciclovir (cont.) Clinical pharmacokinetics • The drug is used topically, orally and i.v. • Little drug is absorbed from topical formulations, and the bioavailability of the oral drug is low (about 20%). • It is widely distributed and crosses the blood-brain barrier. • It is excreted in the urine and in lactating women in the breast milk. Aciclovir (cont.) Therapeutic uses • It is the drug of first choice for Herpes simplex and zoster infections, because of the great efficacy and lower toxicity than the alternatives. • The drug has little activity against cytomegalovirus or Epstein-Barr virus. Aciclovir (cont.) Therapeutic uses • Herpes simplex infections of skin, mucous membranes and cornea • Life-threatening Herpes simplex infections; aciclovir i.v. reduces mortality • Herpes zoster that is less sensitive to aciclovir than H. simplex .It is used for early topic or oral treatment of zoster; aciclovir i.v. is used for life-threatening zoster infections as pneumonia Aciclovir (cont.) Adverse effects • Renal impairment: mainly in high i.v. doses in dehydrated patients • Local inflammation following extravascular administration • Encephalopathy: mainly in high i.v. doses Zidovudine (AZT) Mode of action • HIV virus is an RNA virus capable of including the synthesis of a DNA transcript of its genome, which can then become integrated into the host cell`s DNA, thereby allowing viral replication. • Synthesis of the initial DNA transcript involves the enzyme reverse transcriptase. Zidovudine (AZT) cont. Mode of action • Zidovudine is a potent inhibitor of reverse transcriptase. • It has relatively specific toxicity for the virus. Zidovudine (AZT) cont. Clinical pharmacokinetics • It is well absorbed from the gut but subject to first-pass metabolism • Bioavailability is about 70% • The drug is widely distributed and crosses the blood-brain barrier • Most of the drug is eliminated by hepatic metabolism, unchanged zidovudine accounting for about 10% of the dose Zidovudine (AZT) cont. Clinical pharmacokinetics • In patients with renal or liver impairment, the drug may accumulate, and doses are usually adjusted in these disease states Zidovudine (AZT) cont. Therapeutic uses • It is used to prolong life patients with AIDS and AIDS-related complex (ACR); it probably does not delay the onset of AIDS in HIV-positive patients • The drug usually produces a rise in CD4 cell counts, but eventual deterioration is usual in spite of zidovudine • In patients with late AIDS it is of little use. Zidovudine (AZT) cont. Adverse effects • Bone marrow toxicity • Polymyositis • Headache and insomnia Zidovudine (AZT) cont. Drug interactions • Paracetamol: the risk of bone marrow suppression may increased • Probenecid Purine and pyrimidine analogues Mode of action • These drugs are effective against DNA viruses • The compounds structurally resemble purine and pyrimidine nucleosides • The resulting DNA molecule is more easily fragmented, leading to transcription errors. • They also inhibit viral DNA polymerase. Purine and pyrimidine analogues Examples and clinical pharmacokinetics • Idoxuridine: it is not absorbed from the gut, and is used topically • Vidarabine: cannot be given orally because it is metabolized in the gut - it is usually given i.v. or topically Purine and pyrimidine analogues Therapeutic uses • Idoxuridine: may be used topically for Herpes simplex and zoster but is too toxic for systemic use and has largely been supplanted by aciclovir • Vidarabine: may be used for lifethreatening systemic Herpes infections Purine and pyrimidine analogues Adverse effects • Idoxuridine: because it is used only topically, severe adverse effects are unusual • Vidarabine: anorexia, nausea, vomiting, diarroea and bone marrow suppression Purine and pyrimidine analogues Drug interactions • The metabolism of vidarabine is inhibited by the xanthine oxidase inhibitor allopurinol, and toxicity may result Ribavirin • It is effective against a wide range of DNA and RNA viruses • The drug may be given by aerosol inhalation, orally or i.v. • Oral biavailabity is about 40% • It readily crosses the blood-brain barrier and has a very large volume of distribution, mainly because of cellular uptake. Ribavirin (cont.) • The drug is eliminated by both metabolism and renal excretion, with a terminal half-life of about 2 weeks Ribavirin (cont.) Therapeutic uses • Respiratory syncytial virus (RSV) infections: bronchiolitis and pneumonia at young children • Influenza A and B • Lassa fever

























































































































![ch 14 remember thing[1]](http://s1.studyres.com/store/data/008375860_1-2c45a3b285ef35d04828b346253789f0-150x150.png)










