* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Presentation - Chronice Myeloid Leukemia
Pharmacokinetics wikipedia , lookup
Drug discovery wikipedia , lookup
Orphan drug wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Adherence (medicine) wikipedia , lookup
Pharmacognosy wikipedia , lookup
National Institute for Health and Care Excellence wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Drug interaction wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Psychopharmacology wikipedia , lookup
Prescription costs wikipedia , lookup
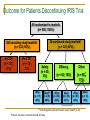
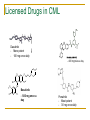
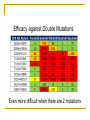
Second and Third Generation Tyrosine Kinase Inhibitors: How do we Choose? Dr Jenny Byrne Nottingham Imatinib is a wonderful drug! IRIS 8-Year Update Overall Survival (Intent-to-Treat) – Imatinib Arm 100 % Alive 90 80 70 i sur ivaa Estimaa ted il l m overall at 8 years was 85% E (93%, considering only CML related deaths) 60 50 40 30 20 Survival: deaths associated with CML Overall Survival 10 0 0 12 24 36 48 60 72 Months Since Randomization 84 96 108 IRIS 8 year Results Imatinib is a great drug …… BUT Not all patients respond well to Imatinib IRIS data excludes patients who discontinued ‘study’ Imatinib Outcome for Patients Discontinuing IRIS Trial All randomized to imatinib (n= 553; 100%) Discontinued study imatinib* (n = 221; 40%) Still receiving study imatinib (n = 332; 60%) In CCR (n = 317; 57%) No CCR (n = 15; 3%) Safety (n = 43; 8%) Alive (n = 17; 40%) Dead** (n = 26; 60%) Efficacy Other (n = 82; 15%) (n = 96 17%) Alive (n = 52; 63%) Dead (n = 30; 37%) ; Alive (n = 81; 84%) **Including primary discontinuation reason ‘Death’ (n=13) *Patients may have continued imatinib off study. Dead (n = 15; 16%) Up to 40% of patients may ‘fail’ Imatinib Imatinib Resistance and Intolerance Some patients do not do well on imatinib... Imatinib resistance may be defined as: Lack/loss of satisfactory response during imatinib therapy or progression from chronic to accelerated phase or from chronic or accelerated to blast phase Imatinib intolerance may be defined as: Side effects requiring either a dose reduction of imatinib to ≤400 mg/day or discontinuation of imatinib due to drugrelated toxicity (Poor adherence / missed doses may also be an issue) Resistance has been defined as primary or acquired (also known as secondary) Primary resistance Acquired resistance Failure to achieve a response Loss of a confirmed response • Primary haematological resistance • failure to achieve a CHR • In chronic phase • failure to return to CP • In advanced disease • Primary cytogenetic resistance • failure to achieve a CCyR or MCyR • Haematological resistance • confirmed loss of a CHR • Cytogenetic resistance • confirmed loss of a MCyR or CCyR • Molecular resistance • increase in BCR-ABL transcripts of more than 1 log • Often assoc with emergence of a bcr-abl mutation Hochhaus et al., Hematol Oncol Clin N Am 2004;18:641–56 BCR/ABL mutations are the most frequently reported mechanism of acquired resistance T315I/A/D F311L/I/V F317L C305S V299L L248V L298V G250E/A/F Q252H/R P296H M237I L324Q G383D M343T K357R K285N E281A E279K T277A L384M L387F/M S348L A350V B M244V D241G F382L A344V E292V Y253H/F E255K/V V289A/I P G321E M388L C M351T/L E355G/D A397P H396R/P E453G/K/A/V E450G/ Q/K Q447R F486S A S438C V379I L3641 F359V/C/D/I 5417Y E459K/Q Adapted from Melo et al (2006) E276G E275K • Kinase domain mutations occur in 50-90% of cases of acquired resistance • Resistance manifests itself through different mechanisms causing • Changes to the stability of the Bcr-Abl conformation • Reduction in binding efficiency of imatinib Branford et al., Blood 2003;102:276–83; Shah et al., Hematol 2005;183–7; Melo et al., Cancer Lett 2006 (in press) There is a Clear Need to Monitor Response to Therapy Carefully: ELN Guidelines 2013 Time Optimal Response Warning Failure Baseline n/a High risk or CCA/Ph+, major route n/a 3 months BCR-ABL1 ≤ 10% and/or Ph+ ≤ 35% BCR-ABL1 >10% and/or Ph+ 36-95% No CHR and/or Ph+ >95% 6 months BCR-ABL1 <1% BCR-ABL1 1-10% and/or Ph+ 0 (CCyR) and/or Ph+ 1-35% BCR-ABL1 >10% and/or Ph+ >35% 12 months BCR-ABL1 ≤ 0.1% (MMR) BCR-ABL1 >1% and/or Ph+ >0 BCR-ABL1 >0.11% Stable or improving ACA/Ph- (-7 or 7q-) Loss of CHR, MMR loss of CCyR, confirmed loss of MMR, mutations, ACA in Ph+ cells Baccarani M, et al. Blood 2013: 122: 872–884 Anytime Importance of achieving optimal response of ≤ 10% BCR-ABLIS at 3 months Early response is predictive of survival Significant difference in 8-year overall survival (OS) rates in a real-world s P < 0.001 • Achieving ≤ 10% BCR-ABL at 3 months correlates with significantly higher rates of improved PFS and OS 1 7 Marin et al. 2012 Important to ‘Get the Drugs in’ to Achieve Optimal responses: Missed Doses Do Matter! Imatinib Dasatinib Dosing in 1st 12 months Achievement of MMR at 12 months (ITT) Achievement of MMR at 12 months (ITT) 100% 125/227 (55.1%) 129/187 (69.0%) 95-99.9% 14/27 (51.9%) 20/29 (69.0%) 80-95% 3/10 (30.0%) 17/37 (45.9%) <80% 6/18 (33.3%) 13/29 (44.8%) Chance of achievement of RQ-PCR <0.1% at 12 months decreases with decreased average dosing nd 2 Which line drug should we choose for our patients? Up to 40% of patients may ‘fail’ 1st line Imatinib Up to 20% may ‘fail’ Nilotinib / dasatinib 1st line TKIs in CML Off patent 2016 Imatinib Development License NICE approved Dasatinib -1st and 2nd line Nilotinib - 1st and 2nd line Bosutinib - 2nd/3rd line if other TKIs not appropriate Ponatinib - T315I or if no other TKI indicated 2000 2005 2010 CDF 2015 Licensed Drugs in CML Dasatinib - More potent - 100 mg once daily - 400 mg twice a day N N NC O Cl O N O NH Cl Bosutinib - 500 mg once a day Ponatinib - Most potent - 30 mg once daily Treating CML is Not Easy! PATIENT FACTORS DISEASE FACTORS Patients are not all the same! Different ages, comorbidities, sensitivity to side effects Compliance CML is not a homogeneous disease! Different biological properties affecting sensitivity to different drugs Different mutations DRUG FACTORS The different drugs are not all equal! Different toxicities Varying efficacy against different mutations Aim is to match the correct patient with the correct drug for their CML! Not that easy as in the UK we are not able to access all the drugs freely What do the Guidelines Say? ELN Guidelines 2013 – 2nd Line Treatment A change of therapy is mandatory for resistance or toxicity If there is intolerance, any other available TKI can be used, including imatinib second-line after a second-generation TKI first-line. For resistance, the logic sequence is: [1] from imatinib to any other available and approved TKI (dasatinib, nilotinib, bosutinib, ponatinib), [2] from nilotinib to other TKIs (dasatinib, bosutinib, ponatinib), and [3] from dasatinib to other TKIs (nilotinib, bosutinib, ponatinib). Regrettably, there are no studies comparing different TKIs in second-line. Therefore, the choice of the second-line TKI is guided by some patient characteristics, mainly age and comorbidities, by the type of side effects with the first TKI, and by the presence of BCR-ABL1 kinase mutations, and also by drug availability and cost, and by doctor experience. No Clear Winner with Respect to Efficacy No trials have directly compared the drugs Factors that need to be borne in mind when choosing 2nd line treatments Any known mutations Known toxicities of the drugs Patient related comorbidities What drugs are funded by the NHS! Efficacy against Different Mutations Efficacy against Double Mutations Even more difficult when there are 2 mutations Side Effects due to the TKIs are caused by their ‘Off-Target’ Effects Most of the drugs have unwanted effects on other cellular proteins leading to their side effects Imatinib (Phos. IC50) PDGFR 72 nM Nilotinib (Phos. IC50) BcrAbl 20 nM Dasatinib (Phos. IC50) Src 0.1 nM Bosutinib (Phos. IC50) Src 3 nM 1. Manley PW, et al. Proc Am Assoc Cancer Res 2007;48:772. 2. Weisberg E, et al. Cancer Cell 2005;7:1129. 3. Remsing Rix LL, et al. Leukemia 2009;23:477. 4. O’Hare T. et al (2009) Cancer Cell. 16: 401-412 > Kit 99 nM > PDGFR 75 nM > BcrAbl 1.8 nM > BcrAbl 85 nM > BcrAbl 221 nM > Kit 209 nM > PDGFR 2.9 nM > PDGFR >3000 > Src >1000 nM > Src >1000 nM > Kit 18 nM > Kit >10000 nM Choice of Drug: Relative Toxicities Imatinib Nilotinib Dasatinib Bosutinib Oedema ++ + + - Diarrhoea ++ + + +++(transient) Rash + ++ + + Headache + ++ ++ + Glucose - ++ - + Lipase - + - + QT prolongation - + + + Hepatotoxicity + + - + Haem toxicity + ++ ++ + Pleural effusions - - ++ (20%) + (3%) Pulm hypertension - - + (0.5%) ?+ PAOD and IHD - + (6%) - - Need to balance risk benefit Choice of Drug - Comorbidities Drug Nilotinib - Worsens blood glucose levels - Cardiovascular risk factors Dasatinib - Pleural effusions - Pulmonary hypertension Try to Avoid in Diabetics Patients with history of cardiovascular problems eg angina, stroke Chronic lung diseases Difficult times… We may not be able to use the drugs we want to due to funding issues NICE & the CDF - Recent changes Currently approved 2nd Line Treatments NICE • Second-line therapy for SMC • Second-line therapy for adults with CP/AP Ph+ CML who are resistant to treatment with standard-dose imatinib or who have imatinib intolerance2 adults with CP Ph+ CML who are resistant to or intolerant of at least one prior treatment including imatinib Nilotinib* Nilotinib5 *If the manufacturer makes nilotinib available with the discount agreed as part of the patient access scheme Dasatinib and high dose Imatinib not approved (2012) NB: Can still get other drugs for certain patients via the Cancer Drugs Fund 1. NICE. Technology appraisal 251. 2. NICE. Technology appraisal 241. 3. SMC. No. 709/11. 4. SMC. No. 01/02. 5. SMC. No. 440/08. Cancer Drugs Fund (CDF) New drugs have to prove their safety, quality and efficacy NICE: clinical effectiveness – how well does something work in comparison with what we already use? AND cost effectiveness – how much more life or quality of life do we get for the extra money spent? A positive NICE appraisal has to be funded by the NHS: as the budget is fixed, something else has to be axed or delayed Main issue with NICE - too slow, so in 2007 government set up CDF to improve access to cancer drugs in the UK Each drug is scored for impact on survival, quality of life, toxicity and ‘unmet need’ (is it the only systemic therapy for that disease?) 14/15 expenditure on November list £390m and £420m in 15/16 84 separate drug indications in the CDF in November 14 and as overspent the lowest scoring 45 indications assessed in this recent prioritisation process and 18 removed (including CML drugs) Due to finish April 2016 – not quite sure what next! Available TKIs: NICE and National CDF 1st Line CP 2nd Line CP 3rd / 4th Line CP Imatinib (NICE) (after IFN) X Nilotinib (NICE) (NICE) X Dasatinib X x If IM intol / ref AND NIL intol (but not refractory) Bosutinib X X If NIL AND DAS intol (but not refractory) Ponatinib X X Unless T315I X Unless T315I Not all patients will respond to 2nd line drugs Nilotinib 2nd Line Responses CHR MCyR CCyR 40- 50% of patients requiring a 2nd line treatment will ‘fail’ There is some evidence for 3rd line treatment using an alternative drug but funding is an issue Other option is a stem cell transplant (which is funded) Bosutinib 3rd Line Results IM + D Resistant (n = 36) IM + D Intolerant (n = 51) IM + NI Resistant (n = 27) 14.8 (2.7–47.3) 28.7 (0.3–49.7) 12.2 (2.0–48.0) 36 50 27 22 (61) 40 (80) 21 (78) 34 38 24 10 (29) 14 (37) 7 (29) Complete 3 (9) 13 (34) 4 (17) Partial 7 (21) 1 (3) 3 (13) 25 47 16 2 (8) 17 (36) 1 (6) Response, n (%) Median follow-up (range), mo Hematologic response Evaluable patients Complete Cytogenetic response Evaluable patients Major Molecular response Evaluable patients Major Khoury et al. Oral presentation 892, ASH 2010. Ponatinib Results Licensed post 1st line if other drugs not suitable Efficacy of 3rd Line Drugs Evidence suggests ponatinib more effective than bostunib Lipton et al (Ariad sponsored) What do the Guidelines Say re 3rd Line? • There are no evidence-based, reliable, specific recommendations for the patients who fail two or even three TKIs. • These patients form a heterogeneous group • Can try any of the remaining TKIs • Consider SCT in eligible patients. • Ponatinib is likely to be more efficient than any other TKI, but there are no comparative studies Summary The second generation drugs are effective for many patients who are resistant to or intolerant of Imatinib Not clear which one is the best Responses may be durable Side effect profiles of the drugs differ and rarely overlap Generally safer than transplants Should be offered to patients who fail / can’t take Imatinib Choice of drug should depend on mutation analysis, side effects & patient comorbidities BUT not all drugs are funded Not a cure all! Some patients won’t respond Limited choice for 3rd line treatment in the UK Transplantation remains an alternative option